Characterization of the clinical and immunologic phenotype and management of 157 individuals with 56 distinct heterozygous NFKB1 mutations
- PMID: 32278790
- PMCID: PMC8246418
- DOI: 10.1016/j.jaci.2019.11.051
Characterization of the clinical and immunologic phenotype and management of 157 individuals with 56 distinct heterozygous NFKB1 mutations
Erratum in
-
Corrigendum.J Allergy Clin Immunol. 2021 Nov;148(5):1345. doi: 10.1016/j.jaci.2021.09.001. J Allergy Clin Immunol. 2021. PMID: 34743834 No abstract available.
Abstract
Background: An increasing number of NFKB1 variants are being identified in patients with heterogeneous immunologic phenotypes.
Objective: To characterize the clinical and cellular phenotype as well as the management of patients with heterozygous NFKB1 mutations.
Methods: In a worldwide collaborative effort, we evaluated 231 individuals harboring 105 distinct heterozygous NFKB1 variants. To provide evidence for pathogenicity, each variant was assessed in silico; in addition, 32 variants were assessed by functional in vitro testing of nuclear factor of kappa light polypeptide gene enhancer in B cells (NF-κB) signaling.
Results: We classified 56 of the 105 distinct NFKB1 variants in 157 individuals from 68 unrelated families as pathogenic. Incomplete clinical penetrance (70%) and age-dependent severity of NFKB1-related phenotypes were observed. The phenotype included hypogammaglobulinemia (88.9%), reduced switched memory B cells (60.3%), and respiratory (83%) and gastrointestinal (28.6%) infections, thus characterizing the disorder as primary immunodeficiency. However, the high frequency of autoimmunity (57.4%), lymphoproliferation (52.4%), noninfectious enteropathy (23.1%), opportunistic infections (15.7%), autoinflammation (29.6%), and malignancy (16.8%) identified NF-κB1-related disease as an inborn error of immunity with immune dysregulation, rather than a mere primary immunodeficiency. Current treatment includes immunoglobulin replacement and immunosuppressive agents.
Conclusions: We present a comprehensive clinical overview of the NF-κB1-related phenotype, which includes immunodeficiency, autoimmunity, autoinflammation, and cancer. Because of its multisystem involvement, clinicians from each and every medical discipline need to be made aware of this autosomal-dominant disease. Hematopoietic stem cell transplantation and NF-κB1 pathway-targeted therapeutic strategies should be considered in the future.
Keywords: NF-κB1-related phenotype; NFKB1 mutation; NFKB1 variant; autosomal dominant; common variable immunodeficiency; reduced penetrance.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
Disclosure of potential conflict of interest: All authors declare that they have no relevant conflicts of interest.
Figures

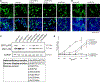
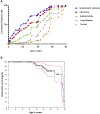
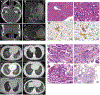

References
-
- Pereira SG, Oakley F. Nuclear factor-κB1: regulation and function. Int J Biochem Cell Biol 2008;40:1425–30. - PubMed
-
- Kaustio M, Haapaniemi E, Göös H, Hautala T, Park G, Syrjänen J, et al. Damaging heterozygous mutations in NFKB1 lead to diverse immunologic phenotypes. J Allergy Clin Immunol 2017;140:782–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

