Development and characterization of CD54-targeted immunoPET imaging in solid tumors
- PMID: 32279097
- PMCID: PMC7554090
- DOI: 10.1007/s00259-020-04784-0
Development and characterization of CD54-targeted immunoPET imaging in solid tumors
Abstract
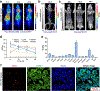
Purpose: Intercellular adhesion molecule-1 (ICAM-1, CD54) is an emerging therapeutic target for a variety of solid tumors including melanoma and anaplastic thyroid cancer (ATC). This study aims to develop an ICAM-1-targeted immuno-positron emission tomography (immunoPET) imaging strategy and assess its diagnostic value in melanoma and ATC models.
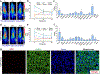
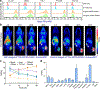
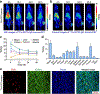
Methods: Flow cytometry was used to screen ICAM-1-positive melanoma and ATC cell lines. Melanoma and ATC models were established using A375 cell line and THJ-16T cell line, respectively. An ICAM-1-specific monoclonal antibody (R6-5-D6) and a nonspecific human IgG were radiolabeled with 64Cu and the diagnostic efficacies were interrogated in tumor-bearing mouse models. Biodistribution and fluorescent imaging studies were performed to confirm the specificity of the ICAM-1-targeted imaging probes.
Results: ICAM-1 was strongly expressed on melanoma and advanced thyroid cancer cell lines. 64Cu-NOTA-ICAM-1 immunoPET imaging efficiently delineated A375 melanomas with a peak tumor uptake of 21.28 ± 6.56 %ID/g (n = 5), significantly higher than that of 64Cu-NOTA-IgG (10.63 ± 2.58 %ID/g, n = 3). Moreover, immunoPET imaging with 64Cu-NOTA-ICAM-1 efficiently visualized subcutaneous and orthotopic ATCs with high clarity and contrast. Fluorescent imaging with IRDye 800CW-ICAM-1 also visualized orthotopic ATCs and the tumor uptake could be blocked by the ICAM-1 parental antibody R6-5-D6, indicating the high specificity of the developed probe. Finally, blocking with the human IgG prolonged the circulation of the 64Cu-NOTA-ICAM-1 in R2G2 mice without compromising the tumor uptake.
Conclusion: ICAM-1-targeted immunoPET imaging could characterize ICAM-1 expression in melanoma and ATC, which holds promise for optimizing ICAM-1-targeted therapies in the future.
Keywords: Companion diagnostics; ICAM-1; ImmunoPET; Melanoma; Thyroid cancer.
Conflict of interest statement
Figures
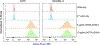

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

