Nintedanib-induced glomerular microangiopathy: a case report
- PMID: 32279192
- PMCID: PMC7502107
- DOI: 10.1007/s13730-020-00474-w
Nintedanib-induced glomerular microangiopathy: a case report
Abstract
Nintedanib, a triple tyrosine kinase inhibitor of vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and fibroblast growth factor receptor, has been used in idiopathic pulmonary fibrosis and adenocarcinoma in advanced non-small cell lung cancer. Although vascular endothelial growth factor inhibitors have been reported to cause endothelial injury and glomerular microangiopathy, nintedanib-induced glomerular microangiopathy has not been reported. A 68-year-old man with a history of primary aldosteronism, idiopathic pulmonary fibrosis, and pleomorphic carcinoma of the lung developed proteinuria and leg edema after nintedanib initiation. Kidney biopsy revealed prominent endothelial and mesangial injury. Proteinuria improved after nintedanib withdrawal. To the best of our knowledge, this is the second case report of nintedanib-induced glomerular microangiopathy. Although the incidence of nephropathy among patients receiving nintedanib is unknown at this moment, we recommend monitoring urinary protein excretion and blood pressure in patients receiving nintedanib and performing kidney biopsy to determine any histopathological change.
Keywords: Glomerular microangiopathy; Nintedanib; Onconephrology; Proteinuria; Pulmonary fibrosis.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures

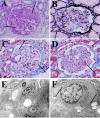
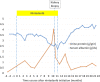
References
-
- Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, Brown KK, Flaherty KR, Noble PW, Raghu G, Brun M, Gupta A, Juhel N, Klüglich M, du Bois RM. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079–1087. doi: 10.1056/NEJMoa1103690. - DOI - PubMed
-
- Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, von Pawel J, Gottfried M, Bondarenko I, Liao M, Gann CN, Barrueco J, Gaschler-Markefski B, LUME-Lung Study Group Novello S Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15:143–155. doi: 10.1016/S1470-2045(13)70586-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

