Liothyronine and Desiccated Thyroid Extract in the Treatment of Hypothyroidism
- PMID: 32279609
- PMCID: PMC7640752
- DOI: 10.1089/thy.2020.0153
Liothyronine and Desiccated Thyroid Extract in the Treatment of Hypothyroidism
Abstract
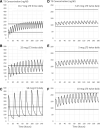
Background: The basis for the treatment of hypothyroidism with levothyroxine (LT4) is that humans activate T4 to triiodothyronine (T3). Thus, while normalizing serum thyrotropin (TSH), LT4 doses should also restore the body's reservoir of T3. However, there is evidence that T3 is not fully restored in LT4-treated patients. Summary: For patients who remain symptomatic on LT4 therapy, clinical guidelines recommend, on a trial basis, therapy with LT4+LT3. Reducing the LT4 dose by 25 mcg/day and adding 2.5-7.5 mcg liothyronine (LT3) once or twice a day is an appropriate starting point. Transient episodes of hypertriiodothyroninemia with these doses of LT4 and LT3 are unlikely to go above the reference range and have not been associated with adverse drug reactions. Trials following almost a 1000 patients for almost 1 year indicate that similar to LT4, therapy with LT4+LT3 can restore euthyroidism while maintaining a normal serum TSH. An observational study of 400 patients with a mean follow-up of ∼9 years did not indicate increased mortality or morbidity risk due to cardiovascular disease, atrial fibrillation, or fractures after adjusting for age when compared with patients taking only LT4. Desiccated thyroid extract (DTE) is a form of combination therapy in which the LT4/LT3 ratio is ∼4:1; the mean daily dose of DTE needed to normalize serum TSH contains ∼11 mcg T3, but some patients may require higher doses. The DTE remains outside formal FDA oversight, and consistency of T4 and T3 contents is monitored by the manufacturers only. Conclusions: Newly diagnosed hypothyroid patients should be treated with LT4. A trial of combination therapy with LT4+LT3 can be considered for those patients who have unambiguously not benefited from LT4.
Keywords: combination therapy; desiccated thyroid extract; hypothyroidism; levothyroxine; triiodothyronine.
Conflict of interest statement
A.C.B. is a consultant for Allergan, Inc. and Synthonics, Inc.; S.P. is chairman of the scientific board for Synthonics, Inc.; and the other authors have nothing to disclose.
Figures
References
-
- Toft AD. 1994. Thyroxine therapy. N Engl J Med 331:174–180 - PubMed
-
- Oppenheimer JH, Braverman LE, Toft A, Jackson IM, Ladenson PW. 1995. A therapeutic controversy. Thyroid hormone treatment: when and what? J Clin Endocrinol Metab 80:2873–2883 - PubMed
-
- Helfand M, Crapo LM. 1990. Monitoring therapy in patients taking levothyroxine. Ann Intern Med 113:450–454 - PubMed
-
- Mandel SJ, Brent GA, Larsen PR. 1993. Levothyroxine therapy in patients with thyroid disease. Ann Intern Med 119:492–502 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

