MMP14 is required for delamination of chick neural crest cells independently of its catalytic activity
- PMID: 32280063
- PMCID: PMC7174838
- DOI: 10.1242/dev.183954
MMP14 is required for delamination of chick neural crest cells independently of its catalytic activity
Abstract
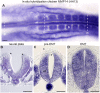
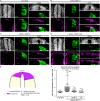
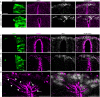
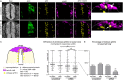
Matrix metalloproteinases have a broad spectrum of substrates ranging from extracellular matrix components and adhesion molecules to chemokines and growth factors. Despite being mostly secreted, MMPs have been detected in the cytosol, the mitochondria or the nucleus. Although most of the attention is focused on their role in matrix remodeling, the diversity of their substrates and their complex trafficking open the possibility for non-canonical functions. Yet in vivo examples and experimental demonstration of the physiological relevance of such activities are rare. Here, we have used chick neural crest (NC) cells, a highly migratory stem cell population likened to invasive cancer cells, as a model for physiological epithelial-mesenchymal transition (EMT). We demonstrate that MMP14 is required for NC delamination. Interestingly, this role is independent of its cytoplasmic tail and of its catalytic activity. Our in vivo data indicate that, in addition to being a late pro-invasive factor, MMP14 is also likely to be an early player, owing to its role in EMT.
Keywords: Delamination; EMT; MMP14; Mitosis; Neural crest; Polarity.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

