Proniosomal Telmisartan Tablets: Formulation, in vitro Evaluation and in vivo Comparative Pharmacokinetic Study in Rabbits
- PMID: 32280201
- PMCID: PMC7127815
- DOI: 10.2147/DDDT.S245013
Proniosomal Telmisartan Tablets: Formulation, in vitro Evaluation and in vivo Comparative Pharmacokinetic Study in Rabbits
Abstract
Objective: The purpose of this study was to prepare proniosomal vesicles of Telmisartan (TEL) to be compressed into tablets which will be further evaluated in vitro and in vivo.
Materials and methods: An experimental design was adopted using surfactants of different HLB values (span 40-brij 35), different cholesterol ratios (20-50%) and different phospholipid types (egg yolk-soyabean). Different responses were measured followed by tablet manufacturing. The highest EE was shown in F3 (85%) while the lowest value was obtained in F7 (8.4%). Finally, zeta potential results were in the range of -0.67 to -27.6 mv. Compressibility percent revealed that F5 showed an excellent flowability characteristic with a value of 9.74±1.61 while F3 and F6 showed good flowability characteristics. By the end of the release, F6 showed approximately 90% drug release.
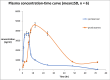
Results: F6 was selected for the in vivo study; Cmax was increased by 1.5-fold while AUC0-∞ also increased significantly by 3-fold when compared with commercial tablet and finally, tmax was increased by 3-fold indicating sustained release pattern. The relative bioavailability was also increased by 3.2-fold.
Conclusion: The results of this study suggested that the formulation of compressed tablets containing more stable proniosomal powder extended the release of TEL and increased its bioavailability as well.
Keywords: EE; TEL; bioavailability; entrapment efficiency; multifactorial design; proniosomal-derived niosomes; sustained-release tablet; telmisartan.
© 2020 Teaima et al.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures






References
-
- Baghel S, Cathcart H, O’reilly NJ. Polymeric amorphous solid dispersions: a review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J Pharm Sci. 2016;105(9):2527–2544. doi: 10.1016/j.xphs.2015.10.008 - DOI - PubMed
-
- Sharma D. Solubility enhancement strategies for poorly water-soluble drugs in solid dispersions: a review. Asian J Pharm. 2016;1.
-
- Dua J, Rana A, Bhandari A. Liposome: methods of preparation and applications. Int J Pharm Stud Res. 2012;3:14–20.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

