Preparation and Characterization of Anti-GPC3 Nanobody Against Hepatocellular Carcinoma
- PMID: 32280214
- PMCID: PMC7125335
- DOI: 10.2147/IJN.S235058
Preparation and Characterization of Anti-GPC3 Nanobody Against Hepatocellular Carcinoma
Abstract
Background: Glypican-3 (GPC3) is a newly identified target molecule for the early diagnosis of hepatocellular carcinoma (HCC), while targeted inhibition of GPC3 signaling may help to control the proliferation and metastasis of HCC cells. The purpose of this study was to prepare the anti-GPC3 nanobody and to investigate the affinity of the anti-GPC3 nanobodies in vitro and the anticancer effects on hepatocellular carcinoma in vivo.
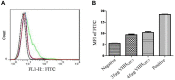
Methods: To screen for unknown anti-GPC3 antibodies, we constructed an antibody phage display library. After three rounds of panning, positive phage clones were identified by enzyme-linked immunosorbent assay (ELISA). Further, the nanobody fusion protein was expressed in E. coli BL21 cells and purified by affinity chromatography. Competitive ELISA and flow cytometry were conducted to confirm the affinity of the anti-GPC3 nanobodies in vitro. The antitumor effects of VHHGPC3 were assessed in vivo.
Results: The results showed that the nanobody VHHGPC3 had specific high-affinity binding to His-GPC3 antigen. Moreover, VHHGPC3 exhibited specific binding to commercial human GPC3 and recognized the surface GPC3 protein of the hepatoma cell line HepG2. Importantly, in vivo study showed that GPC3 nanobody suppresses the growth of HepG2 and improves the survival rate of tumor mice.
Discussion: In summary, our new anti-GPC3 nanobody suggests a strong application potential for targeted therapy of liver cancer.
Keywords: GPC3; nanobody; phage display library; selection.
© 2020 Xia et al.
Conflict of interest statement
The authors declare no conflict of interest in this work.
Figures






References
-
- Cao H, Phan H, Yang L. Improved chemotherapy for hepatocellular carcinoma. Anticancer Res. 2012;32(4):1379–1386. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

