Changing Times for CLN2 Disease: The Era of Enzyme Replacement Therapy
- PMID: 32280231
- PMCID: PMC7127909
- DOI: 10.2147/TCRM.S241048
Changing Times for CLN2 Disease: The Era of Enzyme Replacement Therapy
Abstract
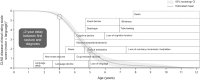
Neuronal ceroid lipofuscinosis type 2 (CLN2 disease) is a progressive neurodegenerative disease that results in early-onset, severe, progressive, neurological disabilities, leading to death in late childhood or early adolescence. Management has relied on symptomatic care, and supportive and palliative strategies, but the approval of the enzyme replacement therapy cerliponase alfa in the USA and Europe in 2017 brought different treatment opportunities. We describe the natural history of CLN2 disease, its diagnosis and management, and the preclinical and clinical development of cerliponase alfa. A PubMed search was undertaken for cerliponase alfa and rhTPP1 to identify preclinical and clinical studies. The hallmark-presenting symptoms of CLN2 disease are unprovoked seizures and a history of language delay, and progression involves motor dysfunction, and cognitive and visual decline. Cerliponase alfa has shown efficacy and tolerability in mouse and canine models of CLN2 disease when delivered intracerebroventricularly. Administration of cerliponase alfa in patients with CLN2 disease has led to significant reductions in the rate of decline of motor and language functions in comparison with a natural history population. The approval of cerliponase alfa has brought a new era for CLN2 disease, highlighting the need to understand different patterns of disease progression and clinical needs in treated patients.
Keywords: TPP1; cerliponase alfa; late infantile; neuronal ceroid lipofuscinosis type 2; seizures.
© 2020 Specchio et al.
Conflict of interest statement
Nicola Specchio has been a consultant for and received grant/research support from BioMarin Pharmaceutical Inc., and reports non-financial support from them during the conduct of the study and grants and personal fees from them outside the submitted work. Nicola Pietrafusa has been a consultant for BioMarin Pharmaceutical Inc. Marina Trivisano has been a consultant for BioMarin Pharmaceutical Inc.
Figures


References
-
- Chang M, Cooper JD, Davidson BL, et al. CLN2 In: Mole S, Williams R, Goebel H, editors. The Neuronal Ceroid Lipofuscinoses (Batten Disease). Oxford: Oxford University Press; 2011:80–109.
Publication types
LinkOut - more resources
Full Text Sources

