Crystal structure of (R, S)-2-hy-droxy-4-(methyl-sulfan-yl)butanoic acid
- PMID: 32280504
- PMCID: PMC7133032
- DOI: 10.1107/S2056989020003138
Crystal structure of (R, S)-2-hy-droxy-4-(methyl-sulfan-yl)butanoic acid
Abstract
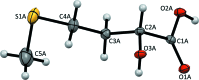
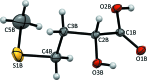
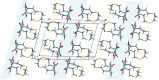
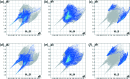
The title compound, a major animal feed supplement, abbreviated as HMTBA and alternatively called dl-me-thio-nine hy-droxy analogue, C5H10O3S, (I), was isolated in pure anhydrous monomeric form. The melting point is 302.5 K and the compound crystallizes in the monoclinic space group P21/c, with two conformationally non-equivalent mol-ecules [(I A ) and (I B )] in the asymmetric unit. The crystal structure is formed by alternating polar and non-polar layers running along the bc plane and features an extensive hydrogen-bonding network within the polar layers. The Hirshfeld surface analysis revealed a significant contribution of non-polar H⋯H and H⋯S inter-actions to the packing forces for both mol-ecules.
Keywords: 2-hydroxy-4-(methylsulfanyl)butanoic acid; CAS 583–91-5; HMTBA; crystal structure; hydrogen bonding; methionine hydroxy analog.
© Mawhinney et al. 2020.
Figures



References
-
- Alagar, M., Krishnakumar, R. V., Mostad, A. & Natarajan, S. (2005). Acta Cryst. E61, o1165–o1167.
-
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
-
- Bhalla, T. C., Kumar, V. & Bhatia, S. K. (2013). Advances in Industrial Biotechnology, edited by R. S. Singh, A. Pandey & C. Larroche, pp. 56–76. Delhi: IK International Publishing House.
-
- Bruker. (2017). APEX3 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Busto, E., Richter, N., Grischek, B. & Kroutil, W. (2014). Chem. Eur. J. 20, 11225–11228. - PubMed
LinkOut - more resources
Full Text Sources
