Norpsilocin: freebase and fumarate salt
- PMID: 32280510
- PMCID: PMC7133046
- DOI: 10.1107/S2056989020004077
Norpsilocin: freebase and fumarate salt
Abstract
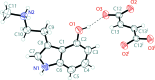
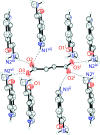
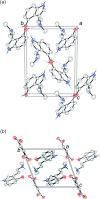
The solid-state structures of the naturally occurring psychoactive tryptamine norpsilocin {4-hy-droxy-N-methyl-tryptamine (4-HO-NMT); systematic name: 3-[2-(methyl-amino)-eth-yl]-1H-indol-4-ol}, C11H14N2O, and its fumarate salt (4-hy-droxy-N-methyl-tryptammonium fumarate; systematic name: bis-{[2-(4-hy-droxy-1H-indol-3-yl)eth-yl]methyl-aza-nium} but-2-enedioate), C11H15N2O+·0.5C4H2O4 2-, are reported. The freebase of 4-HO-NMT has a single mol-ecule in the asymmetric unit joined together by N-H⋯O and O-H⋯O hydrogen bonds in a two-dimensional network parallel to the (100) plane. The ethyl-amine arm of the tryptamine is modeled as a two-component disorder with a 0.895 (3) to 0.105 (3) occupancy ratio. The fumarate salt of 4-HO-NMT crystallizes with a tryptammonium cation and one half of a fumarate dianion in the asymmetric unit. The ions are joined together by N-H⋯O and O-H⋯O hydrogen bonds to form a three-dimensional framework, as well as π-π stacking between the six-membered rings of inversion-related indoles (symmetry operation: 2 - x, 1 - y, 2 - z).
Keywords: crystal structure; hydrogen bonding; indoles; tryptamines.
© Chadeayne et al. 2020.
Figures

References
-
- Bruker (2018). APEX3, SAINT, and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Chadeayne, A. R., Golen, J. A. & Manke, D. R. (2019b). IUCrData, 4, x190962.
-
- Chadeayne, A. R., Golen, J. A. & Manke, D. R. (2019c). Psychedelic Science Review. https://psychedelicreview.com/the-crystal-structure-of-4-aco-dmt-fumarate/
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
