Preemptive Treatment With Elbasvir and Grazoprevir for Hepatitis C-Viremic Donor to Uninfected Recipient Kidney Transplantation
- PMID: 32280841
- PMCID: PMC7136432
- DOI: 10.1016/j.ekir.2020.01.001
Preemptive Treatment With Elbasvir and Grazoprevir for Hepatitis C-Viremic Donor to Uninfected Recipient Kidney Transplantation
Abstract
Introduction: Long wait times for kidney transplants have prompted investigation into strategies to decrease the discarding of potentially viable organs. Recent reports suggest that kidneys from hepatitis C virus (HCV)-infected donors may be transplanted into HCV-naive donors followed by direct-acting antiviral therapy.
Methods: This was a pilot clinical trial to transplant kidneys from HCV-infected donors into HCV-naive recipients with preemptive use of elbasvir and grazoprevir for 12 weeks. The primary outcome was sustained virologic response 12 weeks after completion of therapy. Secondary outcomes were safety, quality of life, and early viral kinetics.
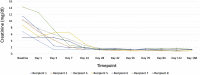
Results: A total of 33 patients were screened, and 8 underwent kidney transplantation from a HCV-viremic donors from August 2017 to March 2019. The median donor kidney donor profile index was 31% (range, 29%-65%), and patients who underwent transplantation waited a median of 6.5 months (range, 1-19 months). None had detectable HCV viremia beyond 2 weeks post-transplantation, and all achieved sustained virologic response 12 weeks after therapy (SVR12). There were no study-related severe adverse events. One patient experienced early graft loss due to venous thrombosis, whereas the remaining 7 patients had excellent allograft function at 6 months.
Conclusion: Preemptive elbasvir and grazoprevir eliminated HCV infection in HCV-naive patients who received a kidney transplant from an HCV-infected donor.
Keywords: direct-acting antivirals; hepatitis C virus; kidney transplantation; organ allocation.
© 2020 International Society of Nephrology. Published by Elsevier Inc.
Figures



Comment in
-
Kidney Transplantation From a Hepatitis C Virus-Infected Donor Into an Uninfected Recipient: Ready for Prime Time?Kidney Int Rep. 2020 Feb 11;5(4):386-388. doi: 10.1016/j.ekir.2020.02.001. eCollection 2020 Apr. Kidney Int Rep. 2020. PMID: 32281985 Free PMC article. No abstract available.
References
-
- Chute D.F., Sise M.E. Effect of the opioid crisis on the donor pool for kidney transplantation: an analysis of national kidney deceased donor trends from 2010-2016. Am J Nephrol. 2018;47:84–93. - PubMed
-
- Levitsky J., Formica R.N., Bloom R.D. The American Society of Transplantation Consensus Conference on the Use of Hepatitis C Viremic Donors in Solid Organ Transplantation. Am J Transplant. 2017;17:2790–2802. - PubMed
-
- Reese P.P., Abt P.L., Blumberg E.A. Transplanting hepatitis C-positive kidneys. N Engl J Med. 2015;373:303–305. - PubMed
-
- Colombo M., Aghemo A., Liu H. Treatment with ledipasvir-sofosbuvir for 12 or 24 weeks in kidney transplant recipients with chronic hepatitis C virus genotype 1 or 4 infection: a randomized trial. Ann Intern Med. 2017;166:109–117. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

