DNAzymes as Catalysts for l-Tyrosine and Amyloid β Oxidation
- PMID: 32280846
- PMCID: PMC7143405
- DOI: 10.1021/acsomega.9b02645
DNAzymes as Catalysts for l-Tyrosine and Amyloid β Oxidation
Abstract
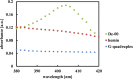
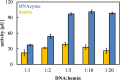
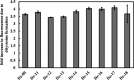
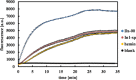
Single-stranded deoxyribonucleic acids have an enormous potential for catalysis by applying tailored sequences of nucleotides for individual reaction conditions and substrates. If such a sequence is guanine-rich, it may arrange into a three-dimensional structure called G-quadruplex and give rise to a catalytically active DNA molecule, a DNAzyme, upon addition of hemin. Here, we present a DNAzyme-mediated reaction, which is the oxidation of l-tyrosine toward dityrosine by hydrogen peroxide. With an optimal stoichiometry between DNA and hemin of 1:10, we report an activity of 101.2 ± 3.5 μUnits (μU) of the artificial DNAzyme Dz-00 compared to 33.0 ± 1.8 μU of free hemin. Exemplarily, DNAzymes may take part in neurodegeneration caused by amyloid beta (Aβ) aggregation due to l-tyrosine oxidation. We show that the natural, human genome-derived DNAzyme In1-sp is able to oxidize Aβ peptides with a 4.6% higher yield and a 33.3% higher velocity of the reaction compared to free hemin. As the artificial DNAzyme Dz-00 is even able to catalyze Aβ peptide oxidation with a 64.2% higher yield and 337.1% higher velocity, an in-depth screening of human genome-derived DNAzymes may identify further candidates with similarly high catalytic activity in Aβ peptide oxidation.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
