The O-GlcNAc modification promotes terminal differentiation of human corneal epithelial cells
- PMID: 32280968
- PMCID: PMC8248940
- DOI: 10.1093/glycob/cwaa033
The O-GlcNAc modification promotes terminal differentiation of human corneal epithelial cells
Abstract
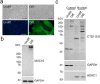
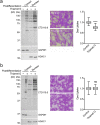
Dynamic modification of nuclear and cytoplasmic proteins with O-linked β-N-acetylglucosamine (O-GlcNAc) plays an important role in orchestrating the transcriptional activity of eukaryotic cells. Here, we report that the O-GlcNAc modification contributes to maintaining ocular surface epithelial homeostasis by promoting mucin biosynthesis and barrier function. We found that induction of human corneal epithelial cell differentiation stimulated the global transfer of O-GlcNAc to both nuclear and cytosolic proteins. Inflammatory conditions, on the other hand, were associated with a reduction in the expression of O-GlcNAc transferase at the ocular surface epithelia. Loss- and gain-of-function studies using small interfering RNA targeting O-GlcNAc transferase, or Thiamet G, a selective inhibitor of O-GlcNAc hydrolase, respectively, revealed that the presence of O-GlcNAc was necessary to promote glycocalyx barrier function. Moreover, we found that Thiamet G triggered a correlative increase in both surface expression of MUC16 and apical epithelial cell area while reducing paracellular permeability. Collectively, these results identify intracellular protein O-glycosylation as a novel pathway responsible for promoting the terminal differentiation of human corneal epithelial cells.
Keywords: O-GlcNAc modification; cell differentiation; cornea; epithelium; transmembrane mucin.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Akimoto Y, Kawakami H, Yamamoto K, Munetomo E, Hida T, Hirano H. 2003. Elevated expression of O-GlcNAc-modified proteins and O-GlcNAc transferase in corneas of diabetic Goto-Kakizaki rats. Invest Ophthalmol Vis Sci. 44:3802–3809. - PubMed
-
- Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. 2003. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 44:2487–2495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

