Effects of extreme potassium stress on blood pressure and renal tubular sodium transport
- PMID: 32281415
- PMCID: PMC7311711
- DOI: 10.1152/ajprenal.00527.2019
Effects of extreme potassium stress on blood pressure and renal tubular sodium transport
Abstract
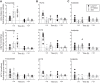
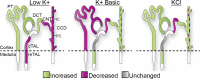
We characterized mouse blood pressure and ion transport in the setting of commonly used rodent diets that drive K+ intake to the extremes of deficiency and excess. Male 129S2/Sv mice were fed either K+-deficient, control, high-K+ basic, or high-KCl diets for 10 days. Mice maintained on a K+-deficient diet exhibited no change in blood pressure, whereas K+-loaded mice developed an ~10-mmHg blood pressure increase. Following challenge with NaCl, K+-deficient mice developed a salt-sensitive 8 mmHg increase in blood pressure, whereas blood pressure was unchanged in mice fed high-K+ diets. Notably, 10 days of K+ depletion induced diabetes insipidus and upregulation of phosphorylated NaCl cotransporter, proximal Na+ transporters, and pendrin, likely contributing to the K+-deficient NaCl sensitivity. While the anionic content with high-K+ diets had distinct effects on transporter expression along the nephron, both K+ basic and KCl diets had a similar increase in blood pressure. The blood pressure elevation on high-K+ diets correlated with increased Na+-K+-2Cl- cotransporter and γ-epithelial Na+ channel expression and increased urinary response to furosemide and amiloride. We conclude that the dietary K+ maneuvers used here did not recapitulate the inverse effects of K+ on blood pressure observed in human epidemiological studies. This may be due to the extreme degree of K+ stress, the low-Na+-to-K+ ratio, the duration of treatment, and the development of other coinciding events, such as diabetes insipidus. These factors must be taken into consideration when studying the physiological effects of dietary K+ loading and depletion.
Keywords: blood pressure; kidney; potassium; sodium transport.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
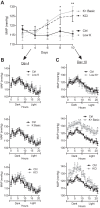
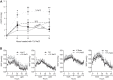
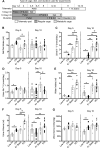



References
-
- Bernabe-Ortiz A, Sal Y Rosas VG, Ponce-Lucero V, Cárdenas MK, Carrillo-Larco RM, Diez-Canseco F, Pesantes MA, Sacksteder KA, Gilman RH, Miranda JJ. Effect of salt substitution on community-wide blood pressure and hypertension incidence. Nat Med 26: 374–378, 2020. doi:10.1038/s41591-020-0754-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

