Challenges and cares to promote rational use of chloroquine and hydroxychloroquine in the management of coronavirus disease 2019 (COVID-19) pandemic: a timely review
- PMID: 32281481
- PMCID: PMC7157945
- DOI: 10.1080/10937404.2020.1752340
Challenges and cares to promote rational use of chloroquine and hydroxychloroquine in the management of coronavirus disease 2019 (COVID-19) pandemic: a timely review
Abstract
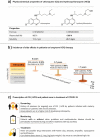
As a result of the 2019 coronavirus disease pandemic (COVID-19), there has been an urgent worldwide demand for treatments. Due to factors such as history of prescription for other infectious diseases, availability, and relatively low cost, the use of chloroquine (CQ) and hydroxychloroquine (HCQ) has been tested in vivo and in vitro for the ability to inhibit the causative virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, even though investigators noted the therapeutic potential of these drugs, it is important to consider the toxicological risks and necessary care for rational use of CQ and HCQ. This study provides information on the main toxicological and epidemiological aspects to be considered for prophylaxis or treatment of COVID-19 using CQ but mainly HCQ, which is a less toxic derivative than CQ, and was shown to produce better results in inhibiting proliferation of SARS-CoV-2 based upon preliminary tests.
Keywords: Toxicology; health care; public health; retinopathy; screening.
Figures
References
-
- Castrejón I., Tani C., Jolly M., Huang A., and Mosca M.. 2014. Indices to assess patients with systemic lupus erythematosus in clinical trials, long-term observational studies, and clinical care. Clin. Exp. Rheumatol. 32 (5 Suppl 85):S85–S95. - PubMed
-
- Gossec L., Molto A., Romand X., Puyraimond-Zemmour D., Lavielle M., Beauvais C., Senbel E., Flipo R. M., Pouplin S., Richez C., et al. 2019. Recommendations for the assessment and optimization of adherence to disease-modifying drugs in chronic inflammatory rheumatic diseases: A process based on literature reviews and expert consensus. Joint Bone Spine 86:13–19. doi:10.1016/j.jbspin.2018.08.006. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
