Quality Assurance of Commercial Insulin Formulations: Novel Assay Using Infrared Spectroscopy
- PMID: 32281880
- PMCID: PMC8252148
- DOI: 10.1177/1932296820913874
Quality Assurance of Commercial Insulin Formulations: Novel Assay Using Infrared Spectroscopy
Abstract
Background: For insulins in commercial formulations, degradation can be observed within the certified shelf life when not stored at recommended conditions. Elevated temperatures and exposure to shear forces can cause changes in the secondary structure of the hormone, leading to a decrease in pharmaceutical potency. International pharmacopoeia recommendations for insulin quality monitoring assays mainly rely on liquid chromatography methods. These methods are unable to distinguish between active and inactive forms, both of which may exist in pharmaceutical insulins exposed to stress conditions.
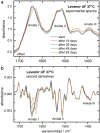
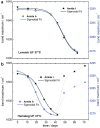
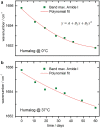
Method: Infrared attenuated total reflection spectroscopy has been used for the analysis of insulin dry film preparations using affordable instrumentation. This method can be applied to either formulated insulin specimens or pure insulins obtained by ultrafiltration. Such samples have been stored under different temperatures (0°C, 20°C, and 37°C), and degradation processes have been monitored up to a period of a few months.
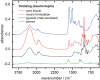
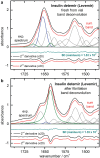
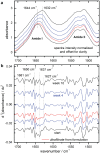
Results: By analyzing specific shifts of absorption bands in the infrared spectra, which are sensitive to the protein secondary structure, even small structural changes in the hormone become evident. Another option is amide I band deconvolution into individual bands, which can be attributed to secondary structure subunits that are part of the insulin tertiary structure.
Conclusion: A novel and innovative method based on infrared attenuated total reflection spectroscopy of insulin dry films is a promising analytical tool for quantifying the degree of insulin degradation, as it provides information on indicating a decrease in biological potency. The established methods for insulin potency assays require animal testing or clamp experiments on people with diabetes.
Keywords: FTIR-ATR spectroscopy; band deconvolution; insulin fibrils; insulin stability; quality control; secondary structure analysis.
Conflict of interest statement
Figures



References
-
- Sarroukh R, Goormaghtigh E, Ruysschart JM, Raussens V. ATR-FTIR: a rejuvenated tool to investigate amyloid proteins. Biochim Biophys Acta. 2013;1828(10):2328-2338. - PubMed
-
- Nielsen L, Khtana R, Coats A, et al.. Effect of environmental factors on the kinetics of insulin fibril formation: elucidation of the molecular mechanism. Biochemistry. 2001;40(20):6036-6046. - PubMed
-
- Beals JM, DeFelippis MR, Kovach PM, Jackson JA. Insulin. In: Crommelin DJA, Sindelar RD, Meibohm B. (eds). Pharmaceutical Biotechnology – Fundamentals and Applications. 4th ed. New York: Springer; 2013:255-275.
-
- Kabsch W, Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features, PDB ID: 3I40. Biopolymers. 1983;22(12):2577-2637. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

