If Human Brain Organoids Are the Answer to Understanding Dementia, What Are the Questions?
- PMID: 32281909
- PMCID: PMC7539594
- DOI: 10.1177/1073858420912404
If Human Brain Organoids Are the Answer to Understanding Dementia, What Are the Questions?
Abstract
Because our beliefs regarding our individuality, autonomy, and personhood are intimately bound up with our brains, there is a public fascination with cerebral organoids, the "mini-brain," the "brain in a dish". At the same time, the ethical issues around organoids are only now being explored. What are the prospects of using human cerebral organoids to better understand, treat, or prevent dementia? Will human organoids represent an improvement on the current, less-than-satisfactory, animal models? When considering these questions, two major issues arise. One is the general challenge associated with using any stem cell-generated preparation for in vitro modelling (challenges amplified when using organoids compared with simpler cell culture systems). The other relates to complexities associated with defining and understanding what we mean by the term "dementia." We discuss 10 puzzles, issues, and stumbling blocks to watch for in the quest to model "dementia in a dish."
Keywords: Alzheimer’s disease; cerebral; cortical; dementia; disease model; induced pluripotent stem cells; neurodegeneration; organoids.
Conflict of interest statement
Figures

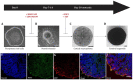



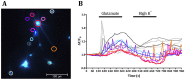
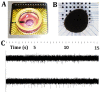
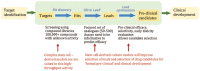
References
-
- Bayne T, Seth AK, Massimini M. 2020. Are there islands of awareness? Trends Neurosci 43(1):6–16. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

