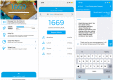
Lifestyle Intervention Enabled by Mobile Technology on Weight Loss in Patients With Nonalcoholic Fatty Liver Disease: Randomized Controlled Trial
- PMID: 32281943
- PMCID: PMC7186867
- DOI: 10.2196/14802
Lifestyle Intervention Enabled by Mobile Technology on Weight Loss in Patients With Nonalcoholic Fatty Liver Disease: Randomized Controlled Trial
Abstract
Background: The prevalence of nonalcoholic fatty liver disease (NAFLD) reaches up to 30% in the Asian adult population, with a higher prevalence in obese patients. Weight reduction is typically recommended for patients at high risk or diagnosed with NAFLD, but is a challenge to achieve.
Objective: We aimed to evaluate the effect of a lifestyle intervention with a mobile app on weight loss in NAFLD patients.
Methods: This prospective randomized controlled trial included 108 adults with NAFLD confirmed by steatosis on ultrasound and a body mass index ≥23 kg/m2 who were recruited from a fatty liver outpatient clinic. The patients were randomly allocated to either a control group (n=53) receiving standard care, consisting of dietary and lifestyle advice by a trained nurse, or an intervention group (n=55) utilizing the Nutritionist Buddy (nBuddy) mobile app in addition to receiving dietary and lifestyle advice by a dietitian. Body weight, alanine aminotransferase (ALT), aspartate aminotransferase (AST), waist circumference, and blood pressure were measured at baseline, and then at 3 and 6 months. Intention-to-treat and per-protocol analyses were used for statistical comparisons.
Results: The intervention group had a 5-fold higher likelihood (relative risk 5.2, P=.003, 95% CI 1.8-15.4) of achieving ≥5% weight loss compared to the control group at 6 months. The intervention group also showed greater reductions in weight (mean 3.2, SD 4.1 kg vs mean 0.5, SD 2.9 kg; P<.001), waist circumference (mean 2.9, SD 5.0 cm vs mean -0.7, SD 4.4 cm; P<.001), systolic blood pressure (mean 12.4, SD 14.8 mmHg vs mean 2.4, SD 12.4 mmHg; P=.003), diastolic blood pressure (mean 6.8, SD 8.9 mmHg vs mean -0.9, SD 10.0 mmHg; P=.001), ALT (mean 33.5, SD 40.4 IU/L vs mean 11.5, SD 35.2 IU/L; P=.004), and AST (mean 17.4, SD 27.5 U/L vs mean 7.4, SD 17.6 IU/L, P=.03) at 6 months.
Conclusions: Lifestyle intervention enabled by a mobile app can be effective in improving anthropometric indices and liver enzymes in patients with NAFLD. This treatment modality has the potential to be extended to a larger population scale.
Trial registration: Australian New Zealand Clinical Trials Registry ACTRN12617001001381; https://tinyurl.com/w9xnfmp.
Keywords: NAFLD; diet; lifestyle intervention; liver enzymes; mHealth; mobile app, weight loss.
©Su Lin Lim, Jolyn Johal, Kai Wen Ong, Chad Yixian Han, Yiong Huak Chan, Yin Mei Lee, Wai Mun Loo. Originally published in JMIR mHealth and uHealth (http://mhealth.jmir.org), 13.04.2020.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011 Aug;34(3):274–285. doi: 10.1111/j.1365-2036.2011.04724.x. doi: 10.1111/j.1365-2036.2011.04724.x. - DOI - DOI - PubMed