Assessment of Acute Wound Healing using the Dorsal Subcutaneous Polyvinyl Alcohol Sponge Implantation and Excisional Tail Skin Wound Models
- PMID: 32281981
- PMCID: PMC7281859
- DOI: 10.3791/60653
Assessment of Acute Wound Healing using the Dorsal Subcutaneous Polyvinyl Alcohol Sponge Implantation and Excisional Tail Skin Wound Models
Abstract
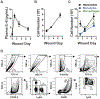
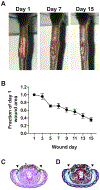
Wound healing is a complex process that requires the orderly progression of inflammation, granulation tissue formation, fibrosis, and resolution. Murine models provide valuable mechanistic insight into these processes; however, no single model fully addresses all aspects of the wound healing response. Instead, it is ideal to use multiple models to address the different aspects of wound healing. Here, two different methods that address diverse aspects of the wound healing response are described. In the first model, polyvinyl alcohol sponges are subcutaneously implanted along the mouse dorsum. Following sponge retrieval, cells can be isolated by mechanical disruption, and fluids can be extracted by centrifugation, thus allowing for a detailed characterization of cellular and cytokine responses in the acute wound environment. A limitation of this model is the inability to assess the rate of wound closure. For this, a tail skin excision model is utilized. In this model, a 10 mm x 3 mm rectangular piece of tail skin is excised along the dorsal surface, near the base of the tail. This model can be easily photographed for planimetric analysis to determine healing rates and can be excised for histological analysis. Both described methods can be utilized in genetically altered mouse strains, or in conjunction with models of comorbid conditions, such as diabetes, aging, or secondary infection, in order to elucidate wound healing mechanisms.
Figures

References
-
- Gottrup F, Agren MS, Karlsmark T Models for use in wound healing research: a survey focusing on in vitro and in vivo adult soft tissue. Wound Repair and Regeneration: Official Publication of the Wound Healing Society [and] the European Tissue Repair Society. 8 (2), 83–96 (2000). - PubMed
-
- Elliot S, Wikramanayake TC, Jozic I, Tomic-Canic M A Modeling Conundrum: Murine Models for Cutaneous Wound Healing. Journal of Investigative Dermatology. 138 (4), 736–740 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
