Sugammadex versus Neostigmine for Reversal of Neuromuscular Blockade and Postoperative Pulmonary Complications (STRONGER): A Multicenter Matched Cohort Analysis
- PMID: 32282427
- PMCID: PMC7864000
- DOI: 10.1097/ALN.0000000000003256
Sugammadex versus Neostigmine for Reversal of Neuromuscular Blockade and Postoperative Pulmonary Complications (STRONGER): A Multicenter Matched Cohort Analysis
Abstract
Background: Five percent of adult patients undergoing noncardiac inpatient surgery experience a major pulmonary complication. The authors hypothesized that the choice of neuromuscular blockade reversal (neostigmine vs. sugammadex) may be associated with a lower incidence of major pulmonary complications.
Methods: Twelve U.S. Multicenter Perioperative Outcomes Group hospitals were included in a multicenter observational matched-cohort study of surgical cases between January 2014 and August 2018. Adult patients undergoing elective inpatient noncardiac surgical procedures with general anesthesia and endotracheal intubation receiving a nondepolarizing neuromuscular blockade agent and reversal were included. Exact matching criteria included institution, sex, age, comorbidities, obesity, surgical procedure type, and neuromuscular blockade agent (rocuronium vs. vecuronium). Other preoperative and intraoperative factors were compared and adjusted in the case of residual imbalance. The composite primary outcome was major postoperative pulmonary complications, defined as pneumonia, respiratory failure, or other pulmonary complications (including pneumonitis; pulmonary congestion; iatrogenic pulmonary embolism, infarction, or pneumothorax). Secondary outcomes focused on the components of pneumonia and respiratory failure.
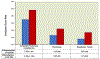
Results: Of 30,026 patients receiving sugammadex, 22,856 were matched to 22,856 patients receiving neostigmine. Out of 45,712 patients studied, 1,892 (4.1%) were diagnosed with the composite primary outcome (3.5% sugammadex vs. 4.8% neostigmine). A total of 796 (1.7%) patients had pneumonia (1.3% vs. 2.2%), and 582 (1.3%) respiratory failure (0.8% vs. 1.7%). In multivariable analysis, sugammadex administration was associated with a 30% reduced risk of pulmonary complications (adjusted odds ratio, 0.70; 95% CI, 0.63 to 0.77), 47% reduced risk of pneumonia (adjusted odds ratio, 0.53; 95% CI, 0.44 to 0.62), and 55% reduced risk of respiratory failure (adjusted odds ratio, 0.45; 95% CI, 0.37 to 0.56), compared to neostigmine.
Conclusions: Among a generalizable cohort of adult patients undergoing inpatient surgery at U.S. hospitals, the use of sugammadex was associated with a clinically and statistically significant lower incidence of major pulmonary complications.
Figures

Comment in
-
Sugammadex and Postoperative Pulmonary Complications: Is Stronger Evidence Required?Anesthesiology. 2020 Jun;132(6):1299-1300. doi: 10.1097/ALN.0000000000003282. Anesthesiology. 2020. PMID: 32371755 No abstract available.
-
Pulmonary Outcomes and Sugammadex versus Neostigmine: Reply.Anesthesiology. 2020 Dec 1;133(6):1312-1313. doi: 10.1097/ALN.0000000000003575. Anesthesiology. 2020. PMID: 33035292 No abstract available.
-
Pulmonary Outcomes and Sugammadex versus Neostigmine: Comment.Anesthesiology. 2020 Dec 1;133(6):1312. doi: 10.1097/ALN.0000000000003574. Anesthesiology. 2020. PMID: 33035295 Free PMC article. No abstract available.
References
-
- Kirmeier E, Eriksson LI, Lewald H, Jonsson Fagerlund M, Hoeft A, Hollmann M, Meistelman C, Hunter JM, Ulm K, Blobner M, POPULAR Contributors: Post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR): a multicentre, prospective observational study. Lancet Respir Med 2019; 7:129–40 - PubMed
-
- Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DA Jr: Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg 2004; 199:531–7 - PubMed
-
- Meara JG, Leather AJM, Hagander L, Alkire BC, Alonso N, Ameh EA, Bickler SW, Conteh L, Dare AJ, Davies J, Mérisier ED, El-Halabi S, Farmer PE, Gawande A, Gillies R, Greenberg SLM, Grimes CE, Gruen RL, Ismail EA, Kamara TB, Lavy C, Lundeg G, Mkandawire NC, Raykar NP, Riesel JN, Rodas E, Rose J, Roy N, Shrime MG, Sullivan R, Verguet S; Watters D; Weiser TG; Wilson IH; Yamey G; Yip W: Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Int J Obstet Anesth 2016; 25:75–8 - PubMed
-
- Nepogodiev D, Martin J, Biccard B, Makupe A, Bhangu A: Global burden of postoperative death. Lancet 2019; 393:401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

