Early prevention of diabetes microvascular complications in people with hyperglycaemia in Europe. ePREDICE randomized trial. Study protocol, recruitment and selected baseline data
- PMID: 32282852
- PMCID: PMC7153858
- DOI: 10.1371/journal.pone.0231196
Early prevention of diabetes microvascular complications in people with hyperglycaemia in Europe. ePREDICE randomized trial. Study protocol, recruitment and selected baseline data
Abstract
Objectives: To assess the effects of early management of hyperglycaemia with antidiabetic drugs plus lifestyle intervention compared with lifestyle alone, on microvascular function in adults with pre-diabetes.
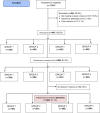
Methods: Trial design: International, multicenter, randomised, partially double-blind, placebo-controlled, clinical trial.
Participants: Males and females aged 45-74 years with IFG, IGT or IFG+IGT, recruited from primary care centres in Australia, Austria, Bulgaria, Greece, Kuwait, Poland, Serbia, Spain and Turkey.
Intervention: Participants were randomized to placebo; metformin 1.700 mg/day; linagliptin 5 mg/day or fixed-dose combination of linagliptin/metformin. All patients were enrolled in a lifestyle intervention program (diet and physical activity). Drug intervention will last 2 years. Primary Outcome: composite end-point of diabetic retinopathy estimated by the Early Treatment Diabetic Retinopathy Study Score, urinary albumin to creatinine ratio, and skin conductance in feet estimated by the sudomotor index. Secondary outcomes in a subsample include insulin sensitivity, beta-cell function, biomarkers of inflammation and fatty liver disease, quality of life, cognitive function, depressive symptoms and endothelial function.
Results: One thousand three hundred ninety one individuals with hyperglycaemia were assessed for eligibility, 424 excluded after screening, 967 allocated to placebo, metformin, linagliptin or to fixed-dose combination of metformin + linagliptin. A total of 809 people (91.1%) accepted and initiated the assigned treatment. Study sample after randomization was well balanced among the four groups. No statistical differences for the main risk factors analysed were observed between those accepting or rejecting treatment initiation. At baseline prevalence of diabetic retinopathy was 4.2%, severe neuropathy 5.3% and nephropathy 5.7%.
Conclusions: ePREDICE is the first -randomized clinical trial with the aim to assess effects of different interventions (lifestyle and pharmacological) on microvascular function in people with pre-diabetes. The trial will provide novel data on lifestyle modification combined with glucose lowering drugs for the prevention of early microvascular complications and diabetes.
Registration: - ClinicalTrials.Gov Identifier: NCT03222765 - EUDRACT Registry Number: 2013-000418-39.
Conflict of interest statement
the authors received funding from Merck Serono GmbH and Boehringer Ingelheim. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials.
Figures
References
-
- World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. WHO, 2006.
-
- Gernstein HC, Santaguida P, Raina P, Morrison KM, Balion C, Hunt D, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res. Clin. Pract. 2007; 78: 305–12 10.1016/j.diabres.2007.05.004 - DOI - PubMed
-
- Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009; 374:1677–86. 10.1016/S0140-6736(09)61457-4 - DOI - PMC - PubMed
-
- Yeboah J, Bertoni AG, Herrington DM, Post WS, Burke GL. Impaired fasting glucose and the risk of incident diabetes mellitus and cardiovascular events in an adult population: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2011; 58:140–6. 10.1016/j.jacc.2011.03.025 - DOI - PMC - PubMed
-
- Heianza Y, Hara S, Arase Y, Saito K, Fujiwara K, Tsuji H, et al. HbA1c 5·7–6·4% and impaired fasting plasma glucose for diagnosis of pre-diabetes and risk of progression to diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet 2011; 378:147–55. 10.1016/S0140-6736(11)60472-8 - DOI - PubMed