Diagnostic Testing for Severe Acute Respiratory Syndrome-Related Coronavirus 2: A Narrative Review
- PMID: 32282894
- PMCID: PMC7170415
- DOI: 10.7326/M20-1301
Diagnostic Testing for Severe Acute Respiratory Syndrome-Related Coronavirus 2: A Narrative Review
Abstract
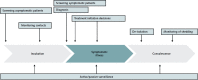
Diagnostic testing to identify persons infected with severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) infection is central to control the global pandemic of COVID-19 that began in late 2019. In a few countries, the use of diagnostic testing on a massive scale has been a cornerstone of successful containment strategies. In contrast, the United States, hampered by limited testing capacity, has prioritized testing for specific groups of persons. Real-time reverse transcriptase polymerase chain reaction-based assays performed in a laboratory on respiratory specimens are the reference standard for COVID-19 diagnostics. However, point-of-care technologies and serologic immunoassays are rapidly emerging. Although excellent tools exist for the diagnosis of symptomatic patients in well-equipped laboratories, important gaps remain in screening asymptomatic persons in the incubation phase, as well as in the accurate determination of live viral shedding during convalescence to inform decisions to end isolation. Many affluent countries have encountered challenges in test delivery and specimen collection that have inhibited rapid increases in testing capacity. These challenges may be even greater in low-resource settings. Urgent clinical and public health needs currently drive an unprecedented global effort to increase testing capacity for SARS-CoV-2 infection. Here, the authors review the current array of tests for SARS-CoV-2, highlight gaps in current diagnostic capacity, and propose potential solutions.
Keywords: Antibodies; Antigens; COVID-19; Cells; Infectious disease immunology; Nucleic acids; Prevention, policy, and public health; Pulmonary diseases; Research laboratories; Upper respiratory tract infections.
Figures

Similar articles
-
Challenges in Laboratory Diagnosis of the Novel Coronavirus SARS-CoV-2.Viruses. 2020 May 26;12(6):582. doi: 10.3390/v12060582. Viruses. 2020. PMID: 32466458 Free PMC article. Review.
-
Laboratory diagnosis of emerging human coronavirus infections - the state of the art.Emerg Microbes Infect. 2020 Dec;9(1):747-756. doi: 10.1080/22221751.2020.1745095. Emerg Microbes Infect. 2020. PMID: 32196430 Free PMC article. Review.
-
Detection of SARS-CoV-2 RNA and Antibodies in Diverse Samples: Protocol to Validate the Sufficiency of Provider-Observed, Home-Collected Blood, Saliva, and Oropharyngeal Samples.JMIR Public Health Surveill. 2020 Apr 24;6(2):e19054. doi: 10.2196/19054. JMIR Public Health Surveill. 2020. PMID: 32310815 Free PMC article.
-
Laboratory preparedness for SARS-CoV-2 testing in India: Harnessing a network of Virus Research & Diagnostic Laboratories.Indian J Med Res. 2020 Feb & Mar;151(2 & 3):216-225. doi: 10.4103/ijmr.IJMR_594_20. Indian J Med Res. 2020. PMID: 32242875 Free PMC article.
-
Molecular Diagnosis of COVID-19: Challenges and Research Needs.Anal Chem. 2020 Aug 4;92(15):10196-10209. doi: 10.1021/acs.analchem.0c02060. Epub 2020 Jul 9. Anal Chem. 2020. PMID: 32573207
Cited by
-
Urgent care center wait times increase for COVID-19 results in August 2020, with rapid testing availability limited.BMC Health Serv Res. 2021 Apr 8;21(1):318. doi: 10.1186/s12913-021-06338-y. BMC Health Serv Res. 2021. PMID: 33832506 Free PMC article.
-
Endourology (Lithiasis). Management, surgical considerations and follow-up of patients in the COVID-19 era.Int Braz J Urol. 2020 Jul;46(suppl.1):39-49. doi: 10.1590/S1677-5538.IBJU.2020.S105. Int Braz J Urol. 2020. PMID: 32568495 Free PMC article. Review.
-
SARS-CoV-2 diagnostic testing alternatives for Latin America.Colomb Med (Cali). 2020 Jun 30;51(2):e4272. doi: 10.25100/cm.v51i2.4272. Colomb Med (Cali). 2020. PMID: 33012887 Free PMC article. Review.
-
The Potential Mechanisms Behind Adverse Effect of Coronavirus Disease-19 on Heart and Liver Damage: A Review.Ethiop J Health Sci. 2024 Jan;34(1):85-100. doi: 10.4314/ejhs.v34i1.10. Ethiop J Health Sci. 2024. PMID: 38957334 Free PMC article. Review.
-
Lessons Learned so Far from the Pandemic: A Review on Pregnants and Neonates with COVID-19.Eurasian J Med. 2020 Jun;52(2):202-210. doi: 10.5152/eurasianjmed.2020.20118. Eurasian J Med. 2020. PMID: 32612432 Free PMC article. Review.
References
-
- World Health Organization. Novel coronavirus (COVID-19) situation. Accessed at https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd. on 24 March 2020.
-
- Mizumoto K, Kagaya K, Chowell G. Early epidemiological assessment of the transmission potential and virulence of coronavirus disease 2019 (COVID-19) in Wuhan City: China, January-February, 2020. Accessed at https://www.medrxiv.org/content/10.1101/2020.02.12.20022434v2. on 24 March 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
