Molecular subtype, biological sex and age shape melanoma tumour evolution
- PMID: 32282938
- PMCID: PMC7613609
- DOI: 10.1111/bjd.19128
Molecular subtype, biological sex and age shape melanoma tumour evolution
Abstract
Background: Many cancer types display sex and age disparity in incidence and outcome. The mutational load of tumours, including melanoma, varies according to sex and age. However, there are no tools to explore systematically whether clinical variables such as age and sex determine the genomic landscape of cancer.
Objectives: To establish a mathematical approach using melanoma mutational data to analyse how sex and age shape the tumour genome.
Methods: We model how age-related (clock-like) somatic mutations that arise during cell division, and extrinsic (environmental ultraviolet radiation) mutations accumulate in cancer genomes.
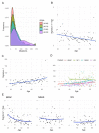
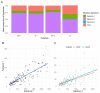
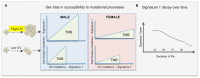
Results: Melanoma is driven primarily by cell-intrinsic age-related mutations and extrinsic ultraviolet radiation-induced mutations, and we show that these mutation types differ in magnitude and chronology and by sex in the distinct molecular melanoma subtypes. Our model confirms that age and sex are determinants of cellular mutation rate, shaping the final mutation composition. We show mathematically for the first time how, similarly to noncancer tissues, melanoma genomes reflect a decline in cell division during ageing. We find that clock-like mutations strongly correlate with the acquisition of ultraviolet-induced mutations, but critically, men present a higher number and rate of cell-division-linked mutations.
Conclusions: These data indicate that the contribution of environmental damage to melanoma likely extends beyond genetic damage to affect cell division. Sex and age determine the final mutational composition of melanoma.
© 2020 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Conflict of interest statement
The authors declare that they have no competing interests
Figures


Comment in
-
The influence of sex, age and sunlight exposure on mutational processes in melanoma.Br J Dermatol. 2021 Feb;184(2):197-198. doi: 10.1111/bjd.19336. Epub 2020 Dec 28. Br J Dermatol. 2021. PMID: 33372289 No abstract available.
Similar articles
-
Impact of Sun Exposure and Tanning Patterns on Next-Generation Sequencing Mutations in Melanoma.J Surg Res. 2020 Oct;254:147-153. doi: 10.1016/j.jss.2020.04.021. Epub 2020 May 21. J Surg Res. 2020. PMID: 32445930
-
Clinical, environmental and histological distribution of BRAF, NRAS and TERT promoter mutations among patients with cutaneous melanoma: a retrospective study of 563 patients.Br J Dermatol. 2021 Mar;184(3):504-513. doi: 10.1111/bjd.19297. Epub 2020 Jul 21. Br J Dermatol. 2021. PMID: 32506424
-
Distinct sets of genetic alterations in melanoma.N Engl J Med. 2005 Nov 17;353(20):2135-47. doi: 10.1056/NEJMoa050092. N Engl J Med. 2005. PMID: 16291983
-
[Melanoma: role of ultraviolet radiation: from physiology to pathology].Presse Med. 2001 Mar 24;30(11):546-51. Presse Med. 2001. PMID: 11317934 Review. French.
-
Acral Melanoma: A Review of Its Pathogenesis, Progression, and Management.Biomolecules. 2025 Jan 14;15(1):120. doi: 10.3390/biom15010120. Biomolecules. 2025. PMID: 39858514 Free PMC article. Review.
Cited by
-
Female Immunity Protects from Cutaneous Squamous Cell Carcinoma.Clin Cancer Res. 2021 Jun 1;27(11):3215-3223. doi: 10.1158/1078-0432.CCR-20-4261. Epub 2021 Apr 1. Clin Cancer Res. 2021. PMID: 33795258 Free PMC article.
-
Shared genetic and epigenetic changes link aging and cancer.Trends Cell Biol. 2022 Apr;32(4):338-350. doi: 10.1016/j.tcb.2022.01.004. Epub 2022 Feb 7. Trends Cell Biol. 2022. PMID: 35144882 Free PMC article. Review.
-
Innovative breakthroughs facilitated by single-cell multi-omics: manipulating natural killer cell functionality correlates with a novel subcategory of melanoma cells.Front Immunol. 2023 Jun 26;14:1196892. doi: 10.3389/fimmu.2023.1196892. eCollection 2023. Front Immunol. 2023. PMID: 37435067 Free PMC article.
-
Gender-Dependent Specificities in Cutaneous Melanoma Predisposition, Risk Factors, Somatic Mutations, Prognostic and Predictive Factors: A Systematic Review.Int J Environ Res Public Health. 2021 Jul 27;18(15):7945. doi: 10.3390/ijerph18157945. Int J Environ Res Public Health. 2021. PMID: 34360236 Free PMC article.
-
UV-Induced Somatic Mutations Driving Clonal Evolution in Healthy Skin, Nevus, and Cutaneous Melanoma.Life (Basel). 2022 Aug 29;12(9):1339. doi: 10.3390/life12091339. Life (Basel). 2022. PMID: 36143375 Free PMC article. Review.
References
-
- Cavanaugh-Hussey MW, Mu EW, Kang S, Balch CM, Wang T. Older Age is Associated with a Higher Incidence of Melanoma Death but a Lower Incidence of Sentinel Lymph Node Metastasis in the SEER Databases (2003–2011) [cited 2018 Nov 14];Ann Surg Oncol. 2015 Jul 5;22(7):2120–6. - PubMed
-
- Li CH, Haider S, Shiah YJ, Thai K, Boutros PC. Sex differences in cancer driver genes and biomarkers. Cancer Res. 2018;78(19):5527–37. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

