Organ-specific metabolic profiles of the liver and kidney during brain death and afterwards during normothermic machine perfusion of the kidney
- PMID: 32282984
- PMCID: PMC7496945
- DOI: 10.1111/ajt.15885
Organ-specific metabolic profiles of the liver and kidney during brain death and afterwards during normothermic machine perfusion of the kidney
Abstract
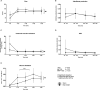
We investigated metabolic changes during brain death (BD) using hyperpolarized magnetic resonance (MR) spectroscopy and ex vivo graft glucose metabolism during normothermic isolated perfused kidney (IPK) machine perfusion. BD was induced in mechanically ventilated rats by inflation of an epidurally placed catheter; sham-operated rats served as controls. Hyperpolarized [1-13 C]pyruvate MR spectroscopy was performed to quantify pyruvate metabolism in the liver and kidneys at 3 time points during BD, preceded by injecting hyperpolarized[1-13 C]pyruvate. Following BD, glucose oxidation was measured using tritium-labeled glucose (d-6-3H-glucose) during IPK reperfusion. Quantitative polymerase chain reaction and biochemistry were performed on tissue/plasma. Immediately following BD induction, lactate increased in both organs (liver: eµd 0.21, 95% confidence interval [CI] [-0.27, -0.15]; kidney: eµd 0.26, 95% CI [-0.40, -0.12]. After 4 hours of BD, alanine production decreased in the kidney (eµd 0.14, 95% CI [0.03, 0.25], P < .05). Hepatic lactate and alanine profiles were significantly different throughout the experiment between groups (P < .01). During IPK perfusion, renal glucose oxidation was reduced following BD vs sham animals (eµd 0.012, 95% CI [0.004, 0.03], P < .001). No differences in enzyme activities were found. Renal gene expression of lactate-transporter MCT4 increased following BD (P < .01). In conclusion, metabolic processes during BD can be visualized in vivo using hyperpolarized magnetic resonance imaging and with glucose oxidation during ex vivo renal machine perfusion. These techniques can detect differences in the metabolic profiles of the liver and kidney following BD.
Keywords: animal models; basic (laboratory) research/science; donors and donation: donation after brain death (DBD); graft survival; kidney (allograft) function/dysfunction; kidney transplantation/nephrology; liver allograft function/dysfunction; liver transplantation/hepatology; organ procurement and allocation; translational research/science.
© 2020 The Authors. American Journal of Transplantation published by Wiley Periodicals LLC on behalf of The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures








References
-
- Organ Procurement and Transplantation Network . https://optn.transplant.hrsa.gov/. Accessed July 6, 2017.
-
- Eurotransplant . https://www.eurotransplant.org/cms/. Accessed February 20, 2018.
-
- Rudge C, Matesanz R, Delmonico FL, Chapman J. International practices of organ donation. Br J Anaesth. 2012;108(Suppl 1):i48‐i55. - PubMed
-
- Mascia L, Bosma K, Pasero D, et al. Ventilatory and hemodynamic management of potential organ donors: an observational survey. Crit Care Med. 2006;34(2):321‐327. - PubMed
-
- Fitzgerald RD, Hieber C, Schweitzer E, et al. Intraoperative catecholamine release in brain‐dead organ donors is not suppressed by administration of fentanyl. Eur J Anaesthesiol. 2003;20:952‐956. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

