Remdesivir and SARS-CoV-2: Structural requirements at both nsp12 RdRp and nsp14 Exonuclease active-sites
- PMID: 32283108
- PMCID: PMC7151495
- DOI: 10.1016/j.antiviral.2020.104793
Remdesivir and SARS-CoV-2: Structural requirements at both nsp12 RdRp and nsp14 Exonuclease active-sites
Abstract
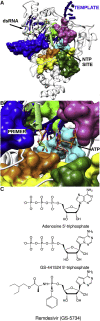
The rapid global emergence of SARS-CoV-2 has been the cause of significant health concern, highlighting the immediate need for antivirals. Viral RNA-dependent RNA polymerases (RdRp) play essential roles in viral RNA synthesis, and thus remains the target of choice for the prophylactic or curative treatment of several viral diseases, due to high sequence and structural conservation. To date, the most promising broad-spectrum class of viral RdRp inhibitors are nucleoside analogues (NAs), with over 25 approved for the treatment of several medically important viral diseases. However, Coronaviruses stand out as a particularly challenging case for NA drug design due to the presence of an exonuclease (ExoN) domain capable of excising incorporated NAs and thus providing resistance to many of these available antivirals. Here we use the available structures of the SARS-CoV RdRp and ExoN proteins, as well as Lassa virus N exonuclease to derive models of catalytically competent SARS-CoV-2 enzymes. We then map a promising NA candidate, GS-441524 (the active metabolite of Remdesivir) to the nucleoside active site of both proteins, identifying the residues important for nucleotide recognition, discrimination, and excision. Interestingly, GS-441524 addresses both enzyme active sites in a manner consistent with significant incorporation, delayed chain termination, and altered excision due to the ribose 1'-CN group, which may account for the increased antiviral effect compared to other available analogues. Additionally, we propose structural and function implications of two previously identified RdRp resistance mutations in relation to resistance against Remdesivir. This study highlights the importance of considering the balance between incorporation and excision properties of NAs between the RdRp and ExoN.
Keywords: COVID-19; Coronavirus; Exonuclease; Mutation; Nucleotide analogue; RNA-Dependent RNA polymerase; Remdesivir; Resistance.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures



References
-
- Booth C.M. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. J. Am. Med. Assoc. 2003;289:2801–2809. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

