Long-term glycemic control and prevention of diabetes complications in vivo using oleic acid-grafted-chitosan‑zinc-insulin complexes incorporated in thermosensitive copolymer
- PMID: 32283211
- PMCID: PMC7299807
- DOI: 10.1016/j.jconrel.2020.04.012
Long-term glycemic control and prevention of diabetes complications in vivo using oleic acid-grafted-chitosan‑zinc-insulin complexes incorporated in thermosensitive copolymer
Abstract
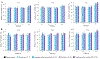
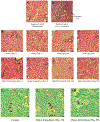
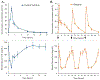
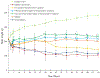
Daily injections for basal insulin therapy are far from ideal resulting in hypo/hyperglycemic episodes associated with fatal complications in type-1 diabetes patients. Here we report a delivery system that provides controlled release of insulin closely mimicking physiological basal insulin requirement for an extended period following a single subcutaneous injection. Stability of insulin was significantly improved by formation of zinc-insulin hexamers, further stabilized by electrostatic complex formation with chitosan polymer. Insulin complexes were homogenously incorporated into PLA-PEG-PLA, a biodegradable thermogel copolymer, that instantaneously forms a subcutaneous gel-depot following injection. Chitosan polymer was hydrophobically modified using oleic acid prior to complex formation with insulin to enable distribution of oleic acid-grafted-chitosan‑zinc-insulin complexes into the hydrophobic core of PLA-PEG-PLA thermogel-copolymer micelles. In vivo, daily administration of marketed long-acting insulin, glargine, resulted in fluctuating blood glucose levels between 91 and 443 mg/dL in type 1 diabetic rats. However, single administration of thermogel copolymeric formulation successfully demonstrated slow diffusion of insulin complexes maintaining peak-free basal insulin level of 21 mU/L for 91 days. Sustained release of basal insulin also correlated with efficient glycemic control (blood glucose <120 mg/dL), prevention of diabetic ketoacidosis and absence of cataract development, unlike other treatment groups. Moreover, there was no sign of inflammation, tissue damage, or collagen deposition around depot site, suggesting exceptional biocompatibility of the formulation for long-term use.
Keywords: Basal insulin; Controlled release; Glycemic control; Hydrophobic modification; Prevention of diabetes complications; Protein stability; Thermosensitive copolymer.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Disclosure
Authors have no competing interests to declare.
Figures


Similar articles
-
Chitosan-zinc-insulin complex incorporated thermosensitive polymer for controlled delivery of basal insulin in vivo.J Control Release. 2012 Oct 28;163(2):145-53. doi: 10.1016/j.jconrel.2012.07.035. Epub 2012 Aug 7. J Control Release. 2012. PMID: 22902516 Free PMC article.
-
Smart Thermosensitive Copolymer Incorporating Chitosan-Zinc-Insulin Electrostatic Complexes for Controlled Delivery of Insulin: Effect of Chitosan Chain Length.Int J Polym Mater. 2020;69(16):1054-1068. doi: 10.1080/00914037.2019.1655750. Epub 2019 Aug 26. Int J Polym Mater. 2020. PMID: 33012880 Free PMC article.
-
Biodegradable triblock copolymer microspheres based on thermosensitive sol-gel transition.Pharm Res. 2004 Feb;21(2):339-43. doi: 10.1023/b:pham.0000016248.30579.2f. Pharm Res. 2004. PMID: 15032317
-
Spotlight on insulin glargine in type 1 and 2 diabetes mellitus.Treat Endocrinol. 2002;1(1):55-8. doi: 10.2165/00024677-200201010-00006. Treat Endocrinol. 2002. PMID: 15765621 Review.
-
Dosing of insulin glargine in the treatment of type 2 diabetes.Clin Ther. 2007 Jun;29(6):987-99. doi: 10.1016/j.clinthera.2007.06.018. Clin Ther. 2007. PMID: 17692716 Review.
Cited by
-
Theranostic advances and the role of molecular imprinting in disease management.iScience. 2025 Mar 10;28(4):112186. doi: 10.1016/j.isci.2025.112186. eCollection 2025 Apr 18. iScience. 2025. PMID: 40224001 Free PMC article. Review.
-
A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics.Int J Mol Sci. 2022 Feb 6;23(3):1851. doi: 10.3390/ijms23031851. Int J Mol Sci. 2022. PMID: 35163773 Free PMC article. Review.
-
Non-Invasive Intranasal Delivery of pApoE2: Effect of Multiple Dosing on the ApoE2 Expression in Mice Brain.Int J Mol Sci. 2023 Aug 21;24(16):13019. doi: 10.3390/ijms241613019. Int J Mol Sci. 2023. PMID: 37629200 Free PMC article.
-
The biological fate of the polymer nanocarrier material monomethoxy poly(ethylene glycol)-block-poly(d,l-lactic acid) in rat.Acta Pharm Sin B. 2021 Apr;11(4):1003-1009. doi: 10.1016/j.apsb.2021.02.018. Epub 2021 Mar 4. Acta Pharm Sin B. 2021. PMID: 33996412 Free PMC article.
-
Synthesis and Characterization of New Biodegradable Injectable Thermosensitive Smart Hydrogels for 5-Fluorouracil Delivery.Int J Mol Sci. 2021 Aug 3;22(15):8330. doi: 10.3390/ijms22158330. Int J Mol Sci. 2021. PMID: 34361098 Free PMC article.
References
-
- Wilcox G, Insulin and insulin resistance., Clin. Biochem. Rev 26 (2005) 19–39. http://www.ncbi.nlm.nih.gov/pubmed/16278749 (accessed September 17, 2019). - PMC - PubMed
-
- Melmed S, Polonsky KS, Larsen PR, Kronenberg H, Williams textbook of endocrinology, 2015.
-
- Coelho JFJ, Ferreira P, Gil MH, New approaches in drug delivery systems: application for diabetes treatment., Infect. Disord. Drug Targets 8 (2008) 119–28. http://www.ncbi.nlm.nih.gov/pubmed/18537707 (accessed September 17, 2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

