Cross-linking of the DNA repair protein O6-alkylguanine DNA alkyltransferase to DNA in the presence of cisplatin
- PMID: 32283495
- PMCID: PMC8177102
- DOI: 10.1016/j.dnarep.2020.102840
Cross-linking of the DNA repair protein O6-alkylguanine DNA alkyltransferase to DNA in the presence of cisplatin
Abstract
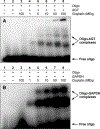
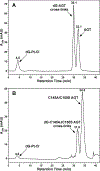
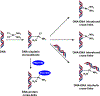
1,1,2,2-cis-diamminedichloroplatinum (II) (cisplatin) is a chemotherapeutic agent widely used in the clinic to treat various cancers. The antitumor activity of cisplatin is generally attributed to its ability to form intrastrand and interstrand DNA-DNA cross-links via sequential platination of two nucleophilic sites within the DNA duplex. However, cisplatin also induces DNA- protein lesions (DPCs) that may contribute to its biological effects due to their ability to block DNA replication and transcription. We previously reported that over 250 nuclear proteins including high mobility group proteins, histone proteins, and elongation factors formed DPCs in human HT1080 cells treated with cisplatin (Ming et al. Chem. Res. Toxicol. 2017, 30, 980-995). Interestingly, cisplatin-induced DNA-protein conjugates were reversed upon heating, by an unknown mechanism. In the present work, DNA repair protein O6-alkylguanine DNA alkyltransferase (AGT) was used as a model to investigate the molecular details of cisplatin-mediated DNA-protein cross-linking and to establish the mechanism of their reversal. We found that AGT is readily cross-linked to DNA in the presence of cisplatin. HPLC-ESI+-MS/MS sequencing of tryptic peptides originating from dG-Pt-AGT complexes revealed that the cross-linking occurred at six sites within this protein including Glu110, Lys125, Cys145, His146, Arg147, and Cys150. Cisplatin-induced Lys-Gua cross-links (1,1-cis-diammine-2-(5-amino-5-carboxypentyl)amino-2-(2'-deoxyguanosine-7-yl)-platinum(II) (dG-Pt-Lys) were detected by HPLC-ESI+-MS/MS of total digests of modified protein in comparison with the corresponding authentic standard. Upon heating, dG-Pt-AGT complexes were subject to platination migration from protein to DNA, forming cis-[Pt(NH3)2{d(GpG)}] cross-links which were detected by HPLC-ESI+-MS/MS. Our results provide a new insight into the mechanism of cisplatin-mediated DNA-protein cross-linking and their dynamic equilibrium with the corresponding DNA-DNA lesions.
Keywords: AGT; Cisplatin; DNA-protein cross-links; Mass spectrometry.
Copyright © 2020. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest No competing interests.
Figures







References
-
- Bain G, Maandag EC, Izon DJ, Amsen D, Kruisbeek AM, Weintraub BC, Krop I, Schlissel MS, Feeney AJ, van Roon M, et al. , E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements, Cell, 79 (1994) 885–892. - PubMed
-
- Dynlacht BD, Regulation of transcription by proteins that control the cell cycle, Nature, 389 (1997) 149–152. - PubMed
-
- Accili D, Arden KC, FoxOs at the crossroads of cellular metabolism, differentiation, and transformation, Cell, 117 (2004) 421–426. - PubMed
-
- Barker S, Weinfeld M, Murray D, DNA-protein crosslinks: their induction, repair, and biological consequences, Mutation Research, 589 (2005) 111–135. - PubMed
-
- Shaham J, Bomstein Y, Meltzer A, Kaufman Z, Palma E, Ribak J, DNA-protein crosslinks, a biomarker of exposure to formaldehyde—in vitro and in vivo studies, Carcinogenesis, 17 (1996) 121–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

