Adiponectin and Its Mimics on Skeletal Muscle: Insulin Sensitizers, Fat Burners, Exercise Mimickers, Muscling Pills … or Everything Together?
- PMID: 32283840
- PMCID: PMC7178193
- DOI: 10.3390/ijms21072620
Adiponectin and Its Mimics on Skeletal Muscle: Insulin Sensitizers, Fat Burners, Exercise Mimickers, Muscling Pills … or Everything Together?
Abstract
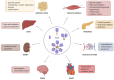
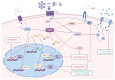
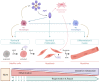
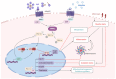
Adiponectin (ApN) is a hormone abundantly secreted by adipocytes and it is known to be tightly linked to the metabolic syndrome. It promotes insulin-sensitizing, fat-burning, and anti-atherosclerotic actions, thereby effectively counteracting several metabolic disorders, including type 2 diabetes, obesity, and cardiovascular diseases. ApN is also known today to possess powerful anti-inflammatory/oxidative and pro-myogenic effects on skeletal muscles exposed to acute or chronic inflammation and injury, mainly through AdipoR1 (ApN specific muscle receptor) and AMP-activated protein kinase (AMPK) pathway, but also via T-cadherin. In this review, we will report all the beneficial and protective properties that ApN can exert, specifically on the skeletal muscle as a target tissue. We will highlight its effects and mechanisms of action, first in healthy skeletal muscle including exercised muscle, and second in diseased muscle from a variety of pathological conditions. In the end, we will go over some of AdipoRs agonists that can be easily produced and administered, and which can greatly mimic ApN. These interesting and newly identified molecules could pave the way towards future therapeutic approaches to potentially prevent or combat not only skeletal muscle disorders but also a plethora of other diseases with sterile inflammation or metabolic dysfunction.
Keywords: AMPK; AdipoRon; Adiponectin; PGC-1α; dystrophinopathies; exercise; inflammation; insulin signaling; obesity; oxidative stress; regeneration; skeletal muscle.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

