Colistin and its role in the Era of antibiotic resistance: an extended review (2000-2019)
- PMID: 32284036
- PMCID: PMC7241451
- DOI: 10.1080/22221751.2020.1754133
Colistin and its role in the Era of antibiotic resistance: an extended review (2000-2019)
Abstract
Increasing antibiotic resistance in multidrug-resistant (MDR) Gram-negative bacteria (MDR-GNB) presents significant health problems worldwide, since the vital available and effective antibiotics, including; broad-spectrum penicillins, fluoroquinolones, aminoglycosides, and β-lactams, such as; carbapenems, monobactam, and cephalosporins; often fail to fight MDR Gram-negative pathogens as well as the absence of new antibiotics that can defeat these "superbugs". All of these has prompted the reconsideration of old drugs such as polymyxins that were reckoned too toxic for clinical use. Only two polymyxins, polymyxin E (colistin) and polymyxin B, are currently commercially available. Colistin has re-emerged as a last-hope treatment in the mid-1990s against MDR Gram-negative pathogens due to the development of extensively drug-resistant GNB. Unfortunately, rapid global resistance towards colistin has emerged following its resurgence. Different mechanisms of colistin resistance have been characterized, including intrinsic, mutational, and transferable mechanisms.In this review, we intend to discuss the progress over the last two decades in understanding the alternative colistin mechanisms of action and different strategies used by bacteria to develop resistance against colistin, besides providing an update about what is previously recognized and what is novel concerning colistin resistance.
Keywords: Colistin; MCR-1; heteroresistance; multidrug resistance; two-component systems.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
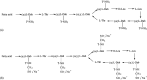



References
-
- Falagas ME, Kasiakou SK.. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005;40(9):1333–1341. - PubMed
-
- Sun J, Zhang H, Liu YH, et al. Towards understanding MCR-like colistin resistance. Trends Microbiol. 2018;26(9):794–808. - PubMed
-
- Jeannot K, Bolard A, Plesiat P.. Resistance to polymyxins in Gram-negative organisms. Int J Antimicrob Agents. 2017;49(5):526–535. - PubMed
-
- Srinivas P, Rivard K.. Polymyxin resistance in Gram-negative pathogens. Curr Infect Dis Rep. 2017;19(11):38. - PubMed
-
- Sherry N, Howden B.. Emerging Gram negative resistance to last-line antimicrobial agents fosfomycin, colistin and ceftazidime-avibactam - epidemiology, laboratory detection and treatment implications. Expert Rev Anti Infect Ther. 2018;16(4):289–306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
