Mycosporine-like amino acids stimulate hyaluronan secretion by up-regulating hyaluronan synthase 2 via activation of the p38/MSK1/CREB/c-Fos/AP-1 axis
- PMID: 32284328
- PMCID: PMC7247295
- DOI: 10.1074/jbc.RA119.011139
Mycosporine-like amino acids stimulate hyaluronan secretion by up-regulating hyaluronan synthase 2 via activation of the p38/MSK1/CREB/c-Fos/AP-1 axis
Abstract
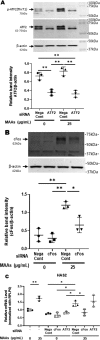
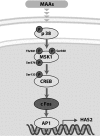
Hyaluronan (HA) is an extracellular matrix glycosaminoglycan that critically supports the physicochemical and mechanical properties of the skin. Here, we demonstrate that mycosporine-like amino acids (MAAs), which typically function as UV-absorbing compounds, can stimulate HA secretion from normal human fibroblasts. MAA-stimulated HA secretion was associated with significantly increased and decreased levels of mRNAs encoding HA synthase 2 (HAS2) and the HA-binding protein involved in HA depolymerization (designated HYBID), respectively. Using immunoblotting, we found that MAAs at 10 and at 25 μg/ml stimulate the phosphorylation of the mitogen-activated protein kinase (MAPK) p38, extracellular signal-regulated kinase (ERK)/c-Jun, and mitogen- and stress-activated protein kinase 1 (MSK1) (at Thr-581, Ser-360, and Ser-376, respectively) and activation of cAMP-responsive element-binding protein (CREB) and activating transcription factor 2 (ATF2), but not phosphorylation of JUN N-terminal kinase (JNK) or NF-κB (at Ser-276 or Ser-536, respectively), and increased c-Fos protein levels. Moreover, a p38-specific inhibitor, but not inhibitors of MAPK/ERK kinase (MEK), JNK, or NF-κB, significantly abrogated the increased expression of HAS2 mRNA, accompanied by significantly decreased MAA-stimulated HA secretion. These results suggested that the p38-MSK1-CREB-c-Fos-transcription factor AP-1 (AP-1) or the p38-ATF2 signaling cascade is responsible for the MAA-induced stimulation of HAS2 gene expression. Of note, siRNA-mediated ATF2 silencing failed to abrogate MAA-stimulated HAS2 expression, and c-Fos silencing abolished the increased expression of HAS2 mRNA. Our findings suggest that MAAs stimulate HA secretion by up-regulating HAS2 mRNA levels through activation of an intracellular signaling cascade consisting of p38, MSK1, CREB, c-Fos, and AP-1.
Keywords: AP-1; CREB; MSK1; amino acid; c-Fos; c-Jun N-terminal kinase (JNK); cell signaling; fibroblast; hyaluronan; hyaluronan synthase 2; mycosporine-like amino acids; p38 MAPK; protein phosphorylation.
© 2020 Terazawa et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures








References
-
- Fisher G. J., Quan T., Purohit T., Shao Y., Cho M. K., He T., Varani J., Kang S., and Voorhees J. J.. 2009) Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 174, 101–114 10.2353/ajpath.2009.080599 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

