Opsonophagocytosis of Chlamydia pneumoniae by Human Monocytes and Neutrophils
- PMID: 32284372
- PMCID: PMC7309617
- DOI: 10.1128/IAI.00087-20
Opsonophagocytosis of Chlamydia pneumoniae by Human Monocytes and Neutrophils
Abstract
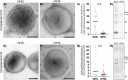
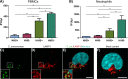
The human respiratory tract pathogen Chlamydia pneumoniae, which causes mild to severe infections, has been associated with the development of chronic inflammatory diseases. To understand the biology of C. pneumoniae infections, several studies have investigated the interaction between C. pneumoniae and professional phagocytes. However, these studies have been conducted under nonopsonizing conditions, making the role of opsonization in C. pneumoniae infections elusive. Thus, we analyzed complement and antibody opsonization of C. pneumoniae and evaluated how opsonization affects chlamydial infectivity and phagocytosis in human monocytes and neutrophils. We demonstrated that IgG antibodies and activation products of complement C3 and C4 are deposited on the surface of C. pneumoniae elementary bodies when incubated in human serum. Complement activation limits C. pneumoniae infectivity in vitro and has the potential to induce bacterial lysis by the formation of the membrane attack complex. Coculture of C. pneumoniae and freshly isolated human leukocytes showed that complement opsonization is superior to IgG opsonization for efficient opsonophagocytosis of C. pneumoniae in monocytes and neutrophils. Neutrophil-mediated phagocytosis of C. pneumoniae was crucially dependent on opsonization, while monocytes retained minor phagocytic potential under nonopsonizing conditions. Complement opsonization significantly enhanced the intracellular neutralization of C. pneumoniae in peripheral blood mononuclear cells and neutrophils and almost abrogated the infectious potential of C. pneumoniae In conclusion, we demonstrated that complements limit C. pneumoniae infection in vitro by interfering with C. pneumoniae entry into permissive cells by direct complement-induced lysis and by tagging bacteria for efficient phagocytosis in both monocytes and neutrophils.
Keywords: Chlamydia pneumoniae; complement; monocytes; neutrophils; opsonization; phagocytosis.
Copyright © 2020 American Society for Microbiology.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

