Detection of Protein Aggregation in Live Plasmodium Parasites
- PMID: 32284383
- PMCID: PMC7269469
- DOI: 10.1128/AAC.02135-19
Detection of Protein Aggregation in Live Plasmodium Parasites
Abstract
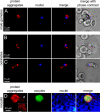
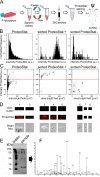
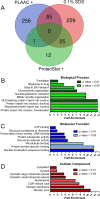
The rapid evolution of resistance in the malaria parasite to every single drug developed against it calls for the urgent identification of new molecular targets. Using a stain specific for the detection of intracellular amyloid deposits in live cells, we have detected the presence of abundant protein aggregates in Plasmodium falciparum blood stages and female gametes cultured in vitro, in the blood stages of mice infected by Plasmodium yoelii, and in the mosquito stages of the murine malaria species Plasmodium berghei Aggregated proteins could not be detected in early rings, the parasite form that starts the intraerythrocytic cycle. A proteomics approach was used to pinpoint actual aggregating polypeptides in functional P. falciparum blood stages, which resulted in the identification of 369 proteins, with roles particularly enriched in nuclear import-related processes. Five aggregation-prone short peptides selected from this protein pool exhibited different aggregation propensity according to Thioflavin-T fluorescence measurements, and were observed to form amorphous aggregates and amyloid fibrils in transmission electron microscope images. The results presented suggest that generalized protein aggregation might have a functional role in malaria parasites. Future antimalarial strategies based on the upsetting of the pathogen's proteostasis and therefore affecting multiple gene products could represent the entry to new therapeutic approaches.
Keywords: malaria; protein aggregation.
Copyright © 2020 American Society for Microbiology.
Figures




References
-
- World Health Organization. 2019. World malaria report, 2019. World Health Organization, Geneva, Switzerland.
-
- Mbengue A, Bhattacharjee S, Pandharkar T, Liu H, Estiu G, Stahelin RV, Rizk SS, Njimoh DL, Ryan Y, Chotivanich K, Nguon C, Ghorbal M, Lopez-Rubio JJ, Pfrender M, Emrich S, Mohandas N, Dondorp AM, Wiest O, Haldar K. 2015. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature 520:683–687. doi:10.1038/nature14412. - DOI - PMC - PubMed

