Chitin perception in plasmodesmata characterizes submembrane immune-signaling specificity in plants
- PMID: 32284410
- PMCID: PMC7196898
- DOI: 10.1073/pnas.1907799117
Chitin perception in plasmodesmata characterizes submembrane immune-signaling specificity in plants
Abstract
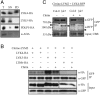
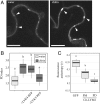
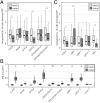
The plasma membrane (PM) is composed of heterogeneous subdomains, characterized by differences in protein and lipid composition. PM receptors can be dynamically sorted into membrane domains to underpin signaling in response to extracellular stimuli. In plants, the plasmodesmal PM is a discrete microdomain that hosts specific receptors and responses. We exploited the independence of this PM domain to investigate how membrane domains can independently integrate a signal that triggers responses across the cell. Focusing on chitin signaling, we found that responses in the plasmodesmal PM require the LysM receptor kinases LYK4 and LYK5 in addition to LYM2. Chitin induces dynamic changes in the localization, association, or mobility of these receptors, but only LYM2 and LYK4 are detected in the plasmodesmal PM. We further uncovered that chitin-induced production of reactive oxygen species and callose depends on specific signaling events that lead to plasmodesmata closure. Our results demonstrate that distinct membrane domains can integrate a common signal with specific machinery that initiates discrete signaling cascades to produce a localized response.
Keywords: immunity; membrane domain; plasmodesmata; receptor signaling.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Ott T., Membrane nanodomains and microdomains in plant-microbe interactions. Curr. Opin. Plant Biol. 40, 82–88 (2017). - PubMed
-
- Gronnier J., Gerbeau-Pissot P., Germain V., Mongrand S., Simon-Plas F., Divide and rule: Plant plasma membrane organization. Trends Plant Sci. 23, 899–917 (2018). - PubMed
-
- Lingwood D., Simons K., Lipid rafts as a membrane-organizing principle. Science 327, 46–50 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

