Colloidal stability of the living cell
- PMID: 32284426
- PMCID: PMC7229749
- DOI: 10.1073/pnas.1914599117
Colloidal stability of the living cell
Abstract
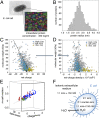
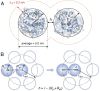
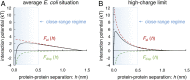
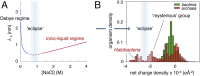
Cellular function is generally depicted at the level of functional pathways and detailed structural mechanisms, based on the identification of specific protein-protein interactions. For an individual protein searching for its partner, however, the perspective is quite different: The functional task is challenged by a dense crowd of nonpartners obstructing the way. Adding to the challenge, there is little information about how to navigate the search, since the encountered surrounding is composed of protein surfaces that are predominantly "nonconserved" or, at least, highly variable across organisms. In this study, we demonstrate from a colloidal standpoint that such a blindfolded intracellular search is indeed favored and has more fundamental impact on the cellular organization than previously anticipated. Basically, the unique polyion composition of cellular systems renders the electrostatic interactions different from those in physiological buffer, leading to a situation where the protein net-charge density balances the attractive dispersion force and surface heterogeneity at close range. Inspection of naturally occurring proteomes and in-cell NMR data show further that the "nonconserved" protein surfaces are by no means passive but chemically biased to varying degree of net-negative repulsion across organisms. Finally, this electrostatic control explains how protein crowding is spontaneously maintained at a constant level through the intracellular osmotic pressure and leads to the prediction that the "extreme" in halophilic adaptation is not the ionic-liquid conditions per se but the evolutionary barrier of crossing its physicochemical boundaries.
Keywords: cellular organization; electrostatics; halophilic adaptation; ion screening; protein–protein interactions.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Nelson D. L., Cox M. M., Lehninger A. L., Lehninger Principles of Biochemistry (Macmillan, 2017).
-
- Valdar W. S., Thornton J. M., Protein-protein interfaces: Analysis of amino acid conservation in homodimers. Proteins 42, 108–124 (2001). - PubMed
-
- Ellis R. J., Macromolecular crowding: Obvious but underappreciated. Trends Biochem. Sci. 26, 597–604 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

