Cell-Penetrating Peptides Enhance the Activity of Human Fibroblast Growth Factor 2 by Prolonging the Retention Time: A New Vision for Drug-Delivery Systems
- PMID: 32284513
- PMCID: PMC7013552
- DOI: 10.3390/ijms21020442
Cell-Penetrating Peptides Enhance the Activity of Human Fibroblast Growth Factor 2 by Prolonging the Retention Time: A New Vision for Drug-Delivery Systems
Abstract
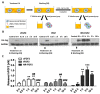
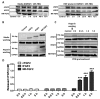
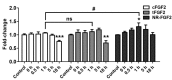
Cell-penetrating peptides (CPPs) are defined by their ability to deliver cargo into cells and have been studied and developed as a promising drug-delivery system (DDS). However, the issue of whether the CPPs that have already entered the cells can be re-released or reused has not been studied. The purpose of this research was to construct CPP-conjugated human fibroblast growth factor 2 (hFGF2) and investigate whether they can be re-released from the cell membrane for reuse. This study combined hFGF2 with Tat or Ara27, a newly developed CPP derived from the zinc knuckle (CCHC-type) family protein of Arabidopsis. Human dermal fibroblast (HDF) was treated with Tat-conjugated hFGF2 (tFGF2) and Ara27-conjugated hFGF2 (NR-FGF2) for both long and short durations, and the effects on cell growth were compared. Furthermore, tFGF2 and NR-FGF2 re-released from the cells were quantified and the effects were evaluated by culturing HDF in a conditioned medium. Interestingly, the proliferation of HDF increased only when NR-FGF2 was treated for 1 h in endocytosis-independent manner. After 1 h, NR-FGF2 was significantly re-released, reaching a maximum concentration at 5 h. Furthermore, increased proliferation of HDF cultured in the conditioned medium containing re-released NR-FGF2 was discovered. While previous studies have focused on the delivery of cargo and its associated applications, this study has revealed that combinations of superior CPPs and therapeutics can be expected to prolong both the retention time and the cell-penetrating capacity, even in the presence of external factors. Therefore, CPPs can be applied in the context of topical drugs and cosmetics as a new DDS approach.
Keywords: Ara27; cell-penetrating peptides; drug-delivery system; fibroblast growth factor 2; re-release.
Conflict of interest statement
The authors declare no conflicts of interest. The founding sponsors had no role in the design of the study; in the collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results. NeoRegen Biotech Co., Ltd. supported the salary for J.S., S.P., and J.L.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

