Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity
- PMID: 32284552
- PMCID: PMC7422941
- DOI: 10.1038/s41551-020-0539-4
Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity
Abstract
Environmental factors are the largest contributors to cardiovascular disease. Here we show that cardiac organoids that incorporate an oxygen-diffusion gradient and that are stimulated with the neurotransmitter noradrenaline model the structure of the human heart after myocardial infarction (by mimicking the infarcted, border and remote zones), and recapitulate hallmarks of myocardial infarction (in particular, pathological metabolic shifts, fibrosis and calcium handling) at the transcriptomic, structural and functional levels. We also show that the organoids can model hypoxia-enhanced doxorubicin cardiotoxicity. Human organoids that model diseases with non-genetic pathological factors could help with drug screening and development.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures


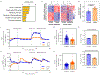
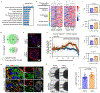


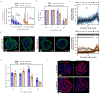
Comment in
-
An in vitro model of myocardial infarction.Nat Biomed Eng. 2020 Apr;4(4):366-367. doi: 10.1038/s41551-020-0550-9. Nat Biomed Eng. 2020. PMID: 32286510 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- P30 GM103342/GM/NIGMS NIH HHS/United States
- U01 DA045300/DA/NIDA NIH HHS/United States
- U54 GM104941/GM/NIGMS NIH HHS/United States
- I01 BX002327/BX/BLRD VA/United States
- R01 HL133308/HL/NHLBI NIH HHS/United States
- T32 HL007260/HL/NHLBI NIH HHS/United States
- R03 DE018741/DE/NIDCR NIH HHS/United States
- P20 GM121342/GM/NIGMS NIH HHS/United States
- P20 GM103499/GM/NIGMS NIH HHS/United States
- P20 GM103444/GM/NIGMS NIH HHS/United States
- P30 CA138313/CA/NCI NIH HHS/United States
- P20 GM103542/GM/NIGMS NIH HHS/United States
- R01 DE021134/DE/NIDCR NIH HHS/United States
- UL1 TR001450/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

