Oxysterols provide innate immunity to bacterial infection by mobilizing cell surface accessible cholesterol
- PMID: 32284563
- PMCID: PMC7442315
- DOI: 10.1038/s41564-020-0701-5
Oxysterols provide innate immunity to bacterial infection by mobilizing cell surface accessible cholesterol
Abstract
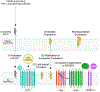
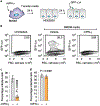
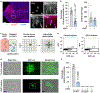
Cholesterol 25-hydroxylase (CH25H) is an interferon-stimulated gene that converts cholesterol to the oxysterol 25-hydroxycholesterol (25HC). Circulating 25HC modulates essential immunological processes including antiviral immunity, inflammasome activation and antibody class switching; and dysregulation of CH25H may contribute to chronic inflammatory disease and cancer. Although 25HC is a potent regulator of cholesterol storage, uptake, efflux and biosynthesis, how these metabolic activities reprogram the immunological state of target cells remains poorly understood. Here, we used recently designed toxin-based biosensors that discriminate between distinct pools of plasma membrane cholesterol to elucidate how 25HC prevents Listeria monocytogenes from traversing the plasma membrane of infected host cells. The 25HC-mediated activation of acyl-CoA:cholesterol acyltransferase (ACAT) triggered rapid internalization of a biochemically defined fraction of cholesterol, termed 'accessible' cholesterol, from the plasma membrane while having little effect on cholesterol in complexes with sphingomyelin. We show that evolutionarily distinct bacterial species, L. monocytogenes and Shigella flexneri, exploit the accessible pool of cholesterol for infection and that acute mobilization of this pool by oxysterols confers immunity to these pathogens. The significance of this signal-mediated membrane remodelling pathway probably extends beyond host defence systems, as several other biologically active oxysterols also mobilize accessible cholesterol through an ACAT-dependent mechanism.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures












Comment in
-
Cholesterol in quarantine.Nat Immunol. 2020 Jul;21(7):716-717. doi: 10.1038/s41590-020-0712-7. Nat Immunol. 2020. PMID: 32514065 No abstract available.
-
Cholesterol access aids pathogen spread.Nat Microbiol. 2020 Jul;5(7):881-882. doi: 10.1038/s41564-020-0744-7. Nat Microbiol. 2020. PMID: 32587375 No abstract available.
References
-
- Schroder K, Hertzog PJ, Ravasi T & Hume DA Interferon-γ: an overview of signals, mechanisms and functions. J. Leukoc. Biol 75, 163–189 (2004). - PubMed
-
- Jouanguy E et al. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nat. Genet 21, 370–378 (1999). - PubMed
-
- Jouanguy E et al. IL-12 and IFN-γ in host defense against mycobacteria and salmonella in mice and men. Curr. Opin. Immunol 11, 346–351 (1999). - PubMed
-
- Radoshevich L & Cossart P Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat. Rev. Microbiol 16, 32–46 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

