Large-scale proteomic analysis of Alzheimer's disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation
- PMID: 32284590
- PMCID: PMC7405761
- DOI: 10.1038/s41591-020-0815-6
Large-scale proteomic analysis of Alzheimer's disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation
Abstract
Our understanding of Alzheimer's disease (AD) pathophysiology remains incomplete. Here we used quantitative mass spectrometry and coexpression network analysis to conduct the largest proteomic study thus far on AD. A protein network module linked to sugar metabolism emerged as one of the modules most significantly associated with AD pathology and cognitive impairment. This module was enriched in AD genetic risk factors and in microglia and astrocyte protein markers associated with an anti-inflammatory state, suggesting that the biological functions it represents serve a protective role in AD. Proteins from this module were elevated in cerebrospinal fluid in early stages of the disease. In this study of >2,000 brains and nearly 400 cerebrospinal fluid samples by quantitative proteomics, we identify proteins and biological processes in AD brains that may serve as therapeutic targets and fluid biomarkers for the disease.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures


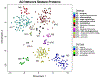









Comment in
-
Large-Scale Proteomics Highlights Glial Role in Neurodegeneration.Cell Metab. 2020 Jul 7;32(1):11-12. doi: 10.1016/j.cmet.2020.06.001. Cell Metab. 2020. PMID: 32640242
References
-
- Prince M , et al. World Alzheimer Report 2015: The Global Impact of Dementia. (Alzheimer’s Disease International, London, 2015).
-
- Clarke C, et al. Correlating transcriptional networks to breast cancer survival: a large-scale coexpression analysis. Carcinogenesis 34, 2300–2308 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG066507/AG/NIA NIH HHS/United States
- R01 AG053960/AG/NIA NIH HHS/United States
- R01 AG057911/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- U01 AG046139/AG/NIA NIH HHS/United States
- RF1 AG051633/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- R01 AG075827/AG/NIA NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
- U01 AG046152/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- R01 AG057339/AG/NIA NIH HHS/United States
- RF1 AG057471/AG/NIA NIH HHS/United States
- R01 AG061800/AG/NIA NIH HHS/United States
- R01 AG058501/AG/NIA NIH HHS/United States
- RF1 AG057440/AG/NIA NIH HHS/United States
- P30 AG066511/AG/NIA NIH HHS/United States
- P50 AG005146/AG/NIA NIH HHS/United States
- RF1 AG054014/AG/NIA NIH HHS/United States
- U01 AG061356/AG/NIA NIH HHS/United States
- U01 AG052411/AG/NIA NIH HHS/United States
- R01 AG072120/AG/NIA NIH HHS/United States
- R01 AG050631/AG/NIA NIH HHS/United States
- R01 AG056533/AG/NIA NIH HHS/United States
- U01 AG046170/AG/NIA NIH HHS/United States
- R01 AG057907/AG/NIA NIH HHS/United States
- I01 BX003853/BX/BLRD VA/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- RF1 AG058501/AG/NIA NIH HHS/United States
- RF1 AG059319/AG/NIA NIH HHS/United States
- RF1 AG051504/AG/NIA NIH HHS/United States
- R01 NS114130/NS/NINDS NIH HHS/United States
- U01 AG061357/AG/NIA NIH HHS/United States
- R01 AG061796/AG/NIA NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- U24 NS072026/NS/NINDS NIH HHS/United States
- U01 AG046161/AG/NIA NIH HHS/United States
- RF1 AG057470/AG/NIA NIH HHS/United States
- K08 NS099474/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

