Orexin signaling in GABAergic lateral habenula neurons modulates aggressive behavior in male mice
- PMID: 32284606
- PMCID: PMC7195257
- DOI: 10.1038/s41593-020-0617-7
Orexin signaling in GABAergic lateral habenula neurons modulates aggressive behavior in male mice
Abstract
Heightened aggression is characteristic of multiple neuropsychiatric disorders and can have various negative effects on patients, their families and the public. Recent studies in humans and animals have implicated brain reward circuits in aggression and suggest that, in subsets of aggressive individuals, domination of subordinate social targets is reinforcing. In this study, we showed that, in male mice, orexin neurons in the lateral hypothalamus activated a small population of glutamic acid decarboxylase 2 (GAD2)-expressing neurons in the lateral habenula (LHb) via orexin receptor 2 (OxR2) and that activation of these GAD2 neurons promoted male-male aggression and conditioned place preference for aggression-paired contexts. Moreover, LHb GAD2 neurons were inhibitory within the LHb and dampened the activity of the LHb as a whole. These results suggest that the orexin system is important for the regulation of inter-male aggressive behavior and provide the first functional evidence of a local inhibitory circuit within the LHb.
Conflict of interest statement
Competing Interests Statement
S.J.R. and M.E.F. have a patent pending (US Patent Application 62/11,233) for the use of OxR2 antagonists to treat aggression.
Figures
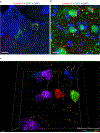
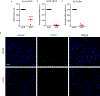
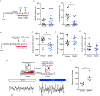
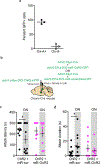
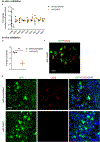
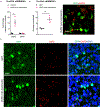


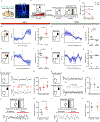


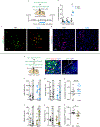

Comment in
-
Behavior: Local Lateral Habenula Interneurons Mediate Aggression.Curr Biol. 2020 Aug 17;30(16):R954-R956. doi: 10.1016/j.cub.2020.06.065. Curr Biol. 2020. PMID: 32810460
References
-
- Miles SR, Menefee DS, Wanner J, Teten Tharp A & Kent TA The Relationship Between Emotion Dysregulation and Impulsive Aggression in Veterans With Posttraumatic Stress Disorder Symptoms. J Interpers Violence 31, 1795–1816 (2016). - PubMed
-
- May ME Aggression as positive reinforcement in people with intellectual disabilities. Research in developmental disabilities 32, 2214–2224 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DP1 DA048931/DA/NIDA NIH HHS/United States
- F31 MH111108/MH/NIMH NIH HHS/United States
- R00 DA045662/DA/NIDA NIH HHS/United States
- R00 DA042111/DA/NIDA NIH HHS/United States
- R01 MH105414/MH/NIMH NIH HHS/United States
- T32 MH087004/MH/NIMH NIH HHS/United States
- R01 MH116445/MH/NIMH NIH HHS/United States
- R21 MH114170/MH/NIMH NIH HHS/United States
- P50 AT008661/AT/NCCIH NIH HHS/United States
- P50 MH096890/MH/NIMH NIH HHS/United States
- R01 MH090264/MH/NIMH NIH HHS/United States
- R01 MH114882/MH/NIMH NIH HHS/United States
- R01 MH051399/MH/NIMH NIH HHS/United States
- K99 DA045662/DA/NIDA NIH HHS/United States
- T32 MH096678/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

