Clinical characteristics of the BREATHE cohort - a real-life study on patients with asthma and COPD
- PMID: 32284828
- PMCID: PMC7144315
- DOI: 10.1080/20018525.2020.1736934
Clinical characteristics of the BREATHE cohort - a real-life study on patients with asthma and COPD
Abstract
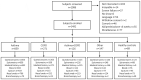
Background: The BREATHE study is a cross-sectional study of real-life patients with asthma and/or COPD in Denmark and Sweden aiming to increase the knowledge across severities and combinations of obstructive airway disease. Design: Patients with suspicion of asthma and/or COPD and healthy controls were invited to participate in the study and had a standard evaluation performed consisting of questionnaires, physical examination, FeNO and lung function, mannitol provocation test, allergy test, and collection of sputum and blood samples. A subgroup of patients and healthy controls had a bronchoscopy performed with a collection of airway samples. Results: The study population consisted of 1403 patients with obstructive airway disease (859 with asthma, 271 with COPD, 126 with concurrent asthma and COPD, 147 with other), and 89 healthy controls (smokers and non-smokers). Of patients with asthma, 54% had moderate-to-severe disease and 46% had mild disease. In patients with COPD, 82% had groups A and B, whereas 18% had groups C and D classified disease. Patients with asthma more frequently had childhood asthma, atopic dermatitis, and allergic rhinitis, compared to patients with COPD, asthma + COPD and Other, whereas FeNO levels were higher in patients with asthma and asthma + COPD compared to COPD and Other (18 ppb and 16 ppb vs 12.5 ppb and 14 ppb, p < 0.001). Patients with asthma, asthma + COPD and Other had higher sputum eosinophilia (1.5%, 1.5%, 1.2% vs 0.75%, respectively, p < 0.001) but lower sputum neutrophilia (39.3, 43.5%, 40.8% vs 66.8%, p < 0.001) compared to patients with COPD. Conclusions: The BREATHE study provides a unique database and biobank with clinical information and samples from 1403 real-life patients with asthma, COPD, and overlap representing different severities of the diseases. This research platform is highly relevant for disease phenotype- and biomarker studies aiming to describe a broad spectrum of obstructive airway diseases.
Keywords: Asthma; COPD; airway hyperresponsiveness; inflammation; real-life population.
© 2020 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
V. Backer has nothing to disclose. D.K. Klein has nothing to disclose. U. Bodtger has nothing to disclose. K. Romberg reports personal fees for lecturing and/or attending advisory board from AstraZeneca, ALK, Boehringer, Chiesi, GSK, Meda, Novartis, and from Teva, outside the submitted work. C. Porsbjerg has nothing to disclose. Jonas S Erjefält has nothing to disclose. Karsten Kristiansen has nothing to disclose. R. Xu has nothing to disclose. Alexander Silberbrandt has nothing to disclose. Laurits Frøssing has nothing to disclose. Morten Hvidtfeldt has nothing to disclose. N. Obling reports personal fees from AstraZeneca, other from Boehringer Ingelheim Denmark A/S, outside the submitted work. Linnea Jarenbäck has nothing to disclose. Abir Nasr has nothing to disclose. Ellen Tufvesson has nothing to disclose. Michiko Mori has nothing to disclose. Matilde Winther-Jensen has nothing to disclose. Lisa Karlsson has nothing to disclose. Ulf Nihlén has nothing to disclose. Thomas Veje Flintegaard has nothing to disclose. L. Bjermer reports personal fees for lecturing and/or attending advisory board from AstraZeneca, ALK, Boehringer, Chiesi, GSK, Novartis, Sanofi and from Teva, outside the submitted work.
Figures
References
-
- Costa DJ, Amouyal M, Lambert P, et al. How representative are clinical study patients with allergic rhinitis in primary care? J Allergy Clin Immunol. 2011;127:920–6.e1. - PubMed
-
- Herland K, Akselsen J-P, Skjonsberg OH, et al. How representative are clinical study patients with asthma or COPD for a larger “real life” population of patients with obstructive lung disease? Respir Med. 2005;99:11–11. - PubMed
-
- Roche N, Reddel H, Martin R, et al. Quality standards for real-world research. Focus on observational database studies of comparative effectiveness. Ann Am Thorac Soc. 2014;11(Suppl 2):S99–104. - PubMed
-
- Saturni S, Bellini F, Braido F, et al. Randomized controlled trials and real life studies. Approaches and methodologies: a clinical point of view. Pulm Pharmacol Ther. 2014;27:129–138. - PubMed
LinkOut - more resources
Full Text Sources
