H3Africa partnerships to empower clinical research sites to generate high-quality biological samples
- PMID: 32284923
- PMCID: PMC7136697
- DOI: 10.4102/ajlm.v9i1.935
H3Africa partnerships to empower clinical research sites to generate high-quality biological samples
Abstract
Background: The Institute of Human Virology Nigeria (IHVN) - Human Heredity and Health in Africa (H3Africa) Biorepository (I-HAB) seeks to provide high-quality biospecimens for research. This depends on the ability of clinical research sites (CRS) - who provide biospecimens - to operate according to well-established industry standards. Yet, standards are often neglected at CRSs located in Africa. Here, I-HAB reports on its four-pronged approach to empower CRSs to prepare high-quality biospecimens for research.
Objectives: I-HAB sought (1) to assess a four-pronged approach to improve biobanking practices and sample quality among CRSs, and (2) to build human capacity.
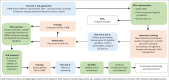
Methods: I-HAB partnered with two H3Africa principal investigators located in Nigeria and Ghana from August 2013 through to May 2017 to debut its four-pronged approach (needs assessment, training and mentorship, pilot, and continuous quality improvement) to empower CRSs to attain high-quality biospecimens.
Results: Close collaborations were instrumental in establishing mutually beneficial and lasting relationships. Improvements during the 12 months of engagement with CRSs involved personnel, procedural, and supply upgrades. In total, 51 staff were trained in over 20 topics. During the pilot, CRSs extracted 50 DNA biospecimens from whole blood and performed quality control. The CRSs shipped extracted DNA to I-HAB and I-HAB that comparatively analysed the DNA. Remediation was achieved via recommendations, training, and mentorship. Preanalytical, analytical and post-analytical processes, standard operating procedures, and workflows were systematically developed.
Conclusion: Partnerships between I-HAB and H3Africa CRSs enabled research sites to produce high-quality biospecimens through needs assessment, training and mentorship, pilot, and continuous monitoring and improvement.
Keywords: Africa; biobank; biotechnology; developing country; training.
© 2020. The Authors.
Conflict of interest statement
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Figures


References
-
- Mayne ES, Croxton T, Abimiku A, et al. . Genes for life: Biobanking for genetic research in Africa. Biopreserv Biobank. 2017;15(2), 93–94. 10.1089/bio.2017.0007 - DOI
-
- Croxton T, Swanepoel C, Musinguzi H, et al. . Lessons learned from biospecimens shipping among the human heredity and health in Africa biorepositories. Biopreserv Biobank. 2017;15(2), 103–110. 10.1089/bio.2017.0009 - DOI
-
- Vaught J, Abayomi A, Peakman T, et al. . Critical issues in international biobanking. Clin Chem [serial online]. 2014;60(11), 1368–1374. [cited 2020 Jan 14] Available from: http://clinchem.aaccjnls.org/content/60/11/1368.long - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
