Quantum biology revisited
- PMID: 32284982
- PMCID: PMC7124948
- DOI: 10.1126/sciadv.aaz4888
Quantum biology revisited
Abstract
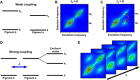
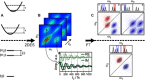
Photosynthesis is a highly optimized process from which valuable lessons can be learned about the operating principles in nature. Its primary steps involve energy transport operating near theoretical quantum limits in efficiency. Recently, extensive research was motivated by the hypothesis that nature used quantum coherences to direct energy transfer. This body of work, a cornerstone for the field of quantum biology, rests on the interpretation of small-amplitude oscillations in two-dimensional electronic spectra of photosynthetic complexes. This Review discusses recent work reexamining these claims and demonstrates that interexciton coherences are too short lived to have any functional significance in photosynthetic energy transfer. Instead, the observed long-lived coherences originate from impulsively excited vibrations, generally observed in femtosecond spectroscopy. These efforts, collectively, lead to a more detailed understanding of the quantum aspects of dissipation. Nature, rather than trying to avoid dissipation, exploits it via engineering of exciton-bath interaction to create efficient energy flow.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Lambert N., Chen Y.-N., Cheng Y.-C., Li C.-M., Chen G.-Y., Nori F., Quantum biology. Nat. Phys. 9, 10–18 (2013).
-
- E. Schrödinger, What is Life? (Cambridge Univ. Press, Cambridge, 1944).
-
- Engel G. S., Calhoun T. R., Read E. L., Ahn T.-K., Mančal T., Cheng Y.-C., Blankenship R. E., Fleming G. R., Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature 446, 782–786 (2007). - PubMed
-
- J. McFadden, J. Al-Khalili, Life on the Edge: The Coming of Age of Quantum Biology. (Broadway Books, 2014).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

