Exosome-based delivery of super-repressor IκBα relieves sepsis-associated organ damage and mortality
- PMID: 32285005
- PMCID: PMC7141819
- DOI: 10.1126/sciadv.aaz6980
Exosome-based delivery of super-repressor IκBα relieves sepsis-associated organ damage and mortality
Abstract
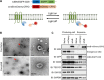
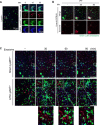
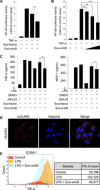
As extracellular vesicles that play an active role in intercellular communication by transferring cellular materials to recipient cells, exosomes offer great potential as a natural therapeutic drug delivery vehicle. The inflammatory responses in various disease models can be attenuated through introduction of super-repressor IκB (srIκB), which is the dominant active form of IκBα and can inhibit translocation of nuclear factor κB into the nucleus. An optogenetically engineered exosome system (EXPLOR) that we previously developed was implemented for loading a large amount of srIκB into exosomes. We showed that intraperitoneal injection of purified srIκB-loaded exosomes (Exo-srIκBs) attenuates mortality and systemic inflammation in septic mouse models. In a biodistribution study, Exo-srIκBs were observed mainly in the neutrophils, and in monocytes to a lesser extent, in the spleens and livers of mice. Moreover, we found that Exo-srIκB alleviates inflammatory responses in monocytic THP-1 cells and human umbilical vein endothelial cells.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures

Similar articles
-
Single-cell analysis of the decidua unveils the mechanism of anti-inflammatory exosomes for chorioamnionitis in nonhuman primates.Sci Adv. 2025 Jul 4;11(27):eadp0467. doi: 10.1126/sciadv.adp0467. Epub 2025 Jul 2. Sci Adv. 2025. PMID: 40601753 Free PMC article.
-
Exosome-Based Delivery of Super-Repressor IκBα Alleviates Alcohol-Associated Liver Injury in Mice.Pharmaceutics. 2023 Feb 14;15(2):636. doi: 10.3390/pharmaceutics15020636. Pharmaceutics. 2023. PMID: 36839957 Free PMC article.
-
Exosome-mediated delivery of super-repressor IκBα alleviates inflammation and joint damages in rheumatoid arthritis.Arthritis Res Ther. 2024 Jan 2;26(1):2. doi: 10.1186/s13075-023-03225-1. Arthritis Res Ther. 2024. PMID: 38167497 Free PMC article.
-
Dual Behavior of Exosomes in Septic Cardiomyopathy.Adv Exp Med Biol. 2017;998:101-112. doi: 10.1007/978-981-10-4397-0_7. Adv Exp Med Biol. 2017. PMID: 28936735 Review.
-
Functional significance of exosomes applied in sepsis: A novel approach to therapy.Biochim Biophys Acta Mol Basis Dis. 2017 Jan;1863(1):292-297. doi: 10.1016/j.bbadis.2016.10.024. Epub 2016 Nov 14. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 27989958 Review.
Cited by
-
Extracellular vesicles in kidneys and their clinical potential in renal diseases.Kidney Res Clin Pract. 2021 Jun;40(2):194-207. doi: 10.23876/j.krcp.20.209. Epub 2021 Apr 13. Kidney Res Clin Pract. 2021. PMID: 33866768 Free PMC article.
-
Exploiting the biogenesis of extracellular vesicles for bioengineering and therapeutic cargo loading.Mol Ther. 2023 May 3;31(5):1231-1250. doi: 10.1016/j.ymthe.2023.02.013. Epub 2023 Feb 20. Mol Ther. 2023. PMID: 36805147 Free PMC article. Review.
-
Bolstering the secretion and bioactivities of umbilical cord MSC-derived extracellular vesicles with 3D culture and priming in chemically defined media.Nano Converg. 2022 Dec 19;9(1):57. doi: 10.1186/s40580-022-00349-z. Nano Converg. 2022. PMID: 36534191 Free PMC article.
-
Tailored Extracellular Vesicles: Novel Tool for Tissue Regeneration.Stem Cells Int. 2022 Jul 29;2022:7695078. doi: 10.1155/2022/7695078. eCollection 2022. Stem Cells Int. 2022. PMID: 35915850 Free PMC article. Review.
-
Hyper-inflammatory responses in COVID-19 and anti-inflammatory therapeutic approaches.BMB Rep. 2022 Jan;55(1):11-19. doi: 10.5483/BMBRep.2022.55.1.152. BMB Rep. 2022. PMID: 34903319 Free PMC article.
References
-
- Boeddha N. P., Schlapbach L. J., Driessen G. J., Herberg J. A., Rivero-Calle I., Cebey-López M., Klobassa D. S., Philipsen R., de Groot R., Inwald D. P., Nadel S., Paulus S., Pinnock E., Secka F., Anderson S. T., Agbeko R. S., Berger C., Fink C. G., Carrol E. D., Zenz W., Levin M., van der Flier M., Martinón-Torres F., Hazelzet J. A., Emonts M.; EUCLIDS consortium , Mortality and morbidity in community-acquired sepsis in European pediatric intensive care units: A prospective cohort study from the European childhood life-threatening infectious disease study (EUCLIDS). Crit. Care 22, 143 (2018). - PMC - PubMed
-
- Mitka M., Drug for severe sepsis is withdrawn from market, fails to reduce mortality. JAMA 306, 2439–2440 (2011). - PubMed
-
- Hotchkiss R. S., Karl I. E., The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348, 138–150 (2003). - PubMed
-
- Iwasaki A., Medzhitov R., Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

