Quantifying the use of connected digital products in clinical research
- PMID: 32285011
- PMCID: PMC7125096
- DOI: 10.1038/s41746-020-0259-x
Quantifying the use of connected digital products in clinical research
Abstract
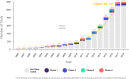
Over recent years, the adoption of connected technologies has grown dramatically, with potential for improving health care delivery, research, and patient experience. Yet, little has been documented about the prevalence and use of connected digital products (e.g., products that capture physiological and behavioral metrics) in formal clinical research. Using 18 years of data from ClinicalTrials.gov, we document substantial growth in the use of connected digital products in clinical trials (~34% CAGR) and show that these products have been used across all phases of research and by a diverse group of trial sponsors. We identify four distinct use cases for how such connected products have been integrated within clinical trial design and suggest implications for various stakeholders engaging in clinical research.
Keywords: Clinical trials; Outcomes research.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsA.C. is the CEO of Elektra Labs. The rest of the authors declare that there are no competing interests.
Figures



References
-
- Garg S, Williams N, Ip A, Dicker A. Clinical integration of digital solutions in health care: an overview of the current landscape of digital technologies in cancer care. JCO Clin. Cancer Inform. 2018;2:2473–4276. - PubMed
LinkOut - more resources
Full Text Sources

