Neuropsychiatric symptoms and cognitive abilities over the initial quinquennium of Parkinson disease
- PMID: 32285645
- PMCID: PMC7187707
- DOI: 10.1002/acn3.51022
Neuropsychiatric symptoms and cognitive abilities over the initial quinquennium of Parkinson disease
Abstract
Objective: To determine the evolution of numerous neuropsychiatric symptoms and cognitive abilities in Parkinson disease from disease onset.
Methods: Prospectively collected, longitudinal (untreated, disease onset to year 5), observational data from Parkinson's Progression Markers Initiative annual visits was used to evaluate prevalence, correlates, and treatment of 10 neuropsychiatric symptoms and cognitive impairment in Parkinson disease participants and matched healthy controls.
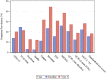
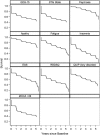
Results: Of 423 Parkinson disease participants evaluated at baseline, 315 (74.5%) were assessed at year 5. Eight neuropsychiatric symptoms studied increased in absolute prevalence by 6.2-20.9% at year 5 relative to baseline, and cognitive impairment increased by 2.7-6.2%. In comparison, the frequency of neuropsychiatric symptoms in healthy controls remained stable or declined over time. Antidepressant and anxiolytic/hypnotic use in Parkinson disease were common at baseline and increased over time (18% to 27% for the former; 13% to 24% for the latter); antipsychotic and cognitive-enhancing medication use was uncommon throughout (2% and 5% of patients at year 5); and potentially harmful anticholinergic medication use was common and increased over time. At year 5 the cross-sectional prevalence for having three or more neuropsychiatric disorders/cognitive impairment was 56% for Parkinson disease participants versus 13% for healthy controls, and by then seven of the examined disorders had either occurred or been treated at some time point in the majority of Parkinson disease patients. Principal component analysis suggested an affective disorder subtype only.
Interpretation: Neuropsychiatric features in Parkinson disease are common from the onset, increase over time, are frequently comorbid, and fluctuate in severity.
© 2020 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association.
Conflict of interest statement
Weintraub reports receiving salary support from the Michael J Fox Foundation during the conduct of the study (Weintraub serves on the Steering Committee of the PPMI Study). Ms. Caspell‐Garcia has nothing to disclose. Simuni reports receiving grant funding from NINDS, MJFF, as well as the Parkinson's Foundation. Additionally, Simuni receives grants from Biogen, Roche, Neuroderm, Sanofi, Sun Pharma, Abbvie, IMPAX, as well as other funding from Acadia, Abbvie, Accorda, Adamas, Allergan, Amneal, Anavex, Aptinyx, Denali, General Electric (GE), Neuroderm, Neurocrine, Sanofi, Sinopia, Sunovion, TEVA, Takeda, Voyager, and US WorldMeds. Cho has nothing to disclose. Coffey received grant funding from the Michael J. Fox Foundation, NHLBI, and NINDS as well as consulting fees from the Michael J. Fox Foundation. Aarsland reports research support or honoraria from AstraZeneca, H. Lundbeck, Novartis Pharmaceuticals and GE Health; served as paid consultant for H. Lundbeck, Biogen, Eisai, Heptares, Sanofi and Mentis Cura; is partly funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London; the views expressed are those of the author and do not necessarily those of the NHR or the Department of Health and Social Care. Alcalay reports receiving personal fees from Sanofi, Roche, Restorbio, Uanssen, and Biogen. Barrett has nothing to disclose. Chahine has nothing to disclose. Eberling has nothing to disclose. Espay reports receiving grants from NIH, Great Lakes Neurotechnologies, the Michael J Fox Foundation, as well as personal fees from Abbvie, TEVA, lmpax, Acadia, Acorda, Cynapsus/Sunovion, Lundbeck, US WorldMeds, UCB, Lippincott Williams & Wilkins, Cambridge University Press, Springer, and lnTrance. Hamilton has nothing to disclose. Hawkins has nothing to disclose. Leverenz reports receiving grants from the Michael J. Fox Foundation, National Institute of Health, as well as other funding from the Veterans Affairs (VA) during the conduct of the study. Additionally, Dr. Leverenz receives grants from the Lewy Body Dementia Association, grants and personal fees from GE 'Healthcare, grants from Genzyme, grants from Alzheimer's Drug Discovery Foundation, personal fees from Eisai, grants and personal fees from Biogen, personal fees from Acadia, personal fees from Aptinyx, grants and personal fees from Sanofi, personal fees from Takeda, and grants from Avid Biopharmaceuticals. Litvan reports receiving grants from the Michael J Fox Foundation during the conduct of the study; grants from the National Institutes of Health, grants from the Parkinson Study Group, grants from the Lewy Body Association, as well as Abbvie, Biogen, Roche, and other funding from the Lundbeck Advisory Board and Sunovion. Richard has nothing to disclose. Rosenthal received support from NIH/NINOS P50NS038377, the Marilyn and Edward Macklin Foundation, and the Michael J. Fox Foundation. She also received an honorarium from the Edmond J. Safra Foundation and from Functional Neuromodulation. Siderowf has nothing to disclose. York has nothing to disclose.
Figures

References
-
- Williams‐Gray C, Evans J, Goris A, et al. The distinct cognitive syndromes of Parkinson's disease: 5 year follow‐up of the CamPaIGN cohort. Brain 2009;132:2958–2969. - PubMed
-
- Aarsland D, Bronnick K, Larsen J, et al. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest Study. Neurology 2009;72:1121–1126. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

