Sex-associated molecular differences for cancer immunotherapy
- PMID: 32286310
- PMCID: PMC7156379
- DOI: 10.1038/s41467-020-15679-x
Sex-associated molecular differences for cancer immunotherapy
Abstract
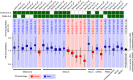
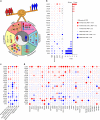
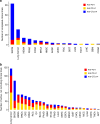
Immune checkpoint blockade therapies have extended patient survival across multiple cancer lineages, but there is a heated debate on whether cancer immunotherapy efficacy is different between male and female patients. We summarize the existing meta-analysis to show inconsistent conclusions for whether gender is associated with the immunotherapy response. We analyze molecular profiling from ICB-treated patients to identify molecular differences for immunotherapy responsiveness. We perform comprehensive analyses for patients from The Cancer Genome Atlas (TCGA) and reveal divergent patterns for sex bias in immune features across multiple cancer types. We further validate our observations in multiple independent data sets. Considering that the majority of clinical trials are in melanoma and lung cancer, meta-analyses that pool multiple cancer types have limitations to discern whether cancer immunotherapy efficacy is different between male and female patients. Future studies should include omics profiling to investigate sex-associated molecular differences in immunotherapy.
Conflict of interest statement
G.B.M. has sponsored research support from AstraZeneca, Critical Outcomes Technology, Karus, Illumina, Immunomet, Nanostring, Tarveda and Immunomet and is on the Scientific Advisory Board for AstraZeneca, Critical Outcomes Technology, ImmunoMet, Ionis, Nuevolution, Symphogen and Tarveda. All other authors declare no competing interests.
Figures

Comment in
-
Gender equality will enhance research around the world.Nature. 2022 Mar;603(7901):362. doi: 10.1038/d41586-022-00722-2. Nature. 2022. PMID: 35292741 No abstract available.
References
-
- Wei, S. C., Duffy, C. R. & Allison, J. P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov.8, 1069–1086 (2018). - PubMed
-
- Klein SL, Flanagan KL. Sex differences in immune responses. Nat. Rev. Cancer. 2016;16:626–638. - PubMed
-
- Conforti F, et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol. 2018;4:1–10. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

