Immunomodulatory role of Interleukin-33 in large vessel vasculitis
- PMID: 32286393
- PMCID: PMC7156501
- DOI: 10.1038/s41598-020-63042-3
Immunomodulatory role of Interleukin-33 in large vessel vasculitis
Abstract
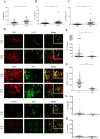
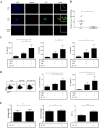
The mechanisms regulating inflammation in large vessels vasculitis (LVV) are poorly understood. Interleukin 33 (IL-33) has been shown to license innate and adaptive immunity by enhancing Th2 cytokines production. We aimed to examine the role of IL-33 in the immunomodulation of T cell activation in LVV. T cell homeostasis and cytokines production were determined in peripheral blood from 52 patients with giant cell arteritis (GCA) and 50 healthy donors (HD), using Luminex assay, flow cytometry, quantitative RT-PCR and by immunofluorescence analysis in inflammatory aorta lesions. We found increased level of IL-33 and its receptor ST2/IL-1R4 in the serum of patient with LVV. Endothelial cells were the main source of IL-33, whereas Th2 cells, Tregs and mast cells (MC) express ST2 in LVV vessels. IL-33 had a direct immunomodulatory impact by increasing Th2 and Tregs. IL-33 and MC further enhanced Th2 and regulatory responses by inducing a 6.1 fold increased proportion of Tregs (p = 0.008). Stimulation of MC by IL-33 increased indoleamine 2 3-dioxygenase (IDO) activity and IL-2 secretion. IL-33 mRNA expression was significantly correlated with the expression of IL-10 and TGF-β within aorta inflammatory lesions. To conclude, our findings suggest that IL-33 may exert a critical immunoregulatory role in promoting Tregs and Th2 cells in LVV.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Saadoun, D. et al. Th1 and Th17 cytokines drive Takayasu Arteritis inflammation. Arthritis Rheumatol. Hoboken NJ, 10.1002/art.39037 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

