C-reactive protein (CRP) recognizes uric acid crystals and recruits proteases C1 and MASP1
- PMID: 32286427
- PMCID: PMC7156728
- DOI: 10.1038/s41598-020-63318-8
C-reactive protein (CRP) recognizes uric acid crystals and recruits proteases C1 and MASP1
Abstract
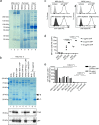
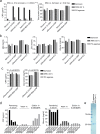
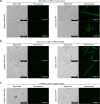
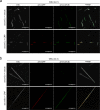
Gout is caused by crystallization of uric acid in the form of monosodium urate (MSU) crystals, which induce a sterile inflammatory response that is hardly distinguishable from microbe-induced inflammatory responses. It is unclear, if MSU crystals (like microbes) are recognized by specific pattern recognition receptors. To identify possible soluble pattern recognition molecules for MSU crystals, we purified MSU-binding proteins from human body fluids. We identified C-reactive protein (CRP) as a major MSU-binding protein. Binding of CRP was strong enough to specifically deplete CRP from human serum. We found that CRP was required for fixation of complement components C1q, C1r, C1s and MASP1. Thus, we have identified a pattern recognition molecule for MSU crystals that links to the activation of complement. Notably, CRP does not show an even binding to the complete surface of the crystals. It rather binds to edges or distinct faces of the crystals.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Byers PH, Ward PA, Kellermeyer RW, Naff GB. Complement as a mediator of inflammation in acute gouty arthritis. II. Biological activities generated from complement by the interaction of serum complement and sodium urate crystals. The Journal of laboratory and clinical medicine. 1973;81:761–769. - PubMed
-
- Naff GB, Byers PH. Complement as a mediator of inflammation in acute gouty arthritis. I. Studies on the reaction between human serum complement and sodium urate crystals. The Journal of laboratory and clinical medicine. 1973;81:747–760. - PubMed
-
- di Giovine FS, Malawista SE, Thornton E, Duff GW. Urate crystals stimulate production of tumor necrosis factor alpha from human blood monocytes and synovial cells. Cytokine mRNA and protein kinetics, and cellular distribution. The Journal of clinical investigation. 1991;87:1375–1381. doi: 10.1172/JCI115142. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

