Altered organization of collagen fibers in the uninvolved human colon mucosa 10 cm and 20 cm away from the malignant tumor
- PMID: 32286443
- PMCID: PMC7156654
- DOI: 10.1038/s41598-020-63368-y
Altered organization of collagen fibers in the uninvolved human colon mucosa 10 cm and 20 cm away from the malignant tumor
Abstract
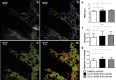
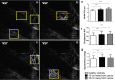
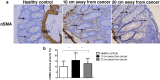
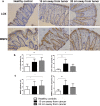
Remodelling of collagen fibers has been described during every phase of cancer genesis and progression. Changes in morphology and organization of collagen fibers contribute to the formation of microenvironment that favors cancer progression and development of metastasis. However, there are only few data about remodelling of collagen fibers in healthy looking mucosa distant from the cancer. Using SHG imaging, electron microscopy and specialized softwares (CT-FIRE, CurveAlign and FiberFit), we objectively visualized and quantified changes in morphology and organization of collagen fibers and investigated possible causes of collagen remodelling (change in syntheses, degradation and collagen cross-linking) in the colon mucosa 10 cm and 20 cm away from the cancer in comparison with healthy mucosa. We showed that in the lamina propria this far from the colon cancer, there were changes in collagen architecture (width, straightness, alignment of collagen fibers and collagen molecules inside fibers), increased representation of myofibroblasts and increase expression of collagen-remodelling enzymes (LOX and MMP2). Thus, the changes in organization of collagen fibers, which were already described in the cancer microenvironment, also exist in the mucosa far from the cancer, but smaller in magnitude.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

