Metabotropic glutamate receptor 5 in bulimia nervosa
- PMID: 32286451
- PMCID: PMC7156702
- DOI: 10.1038/s41598-020-63389-7
Metabotropic glutamate receptor 5 in bulimia nervosa
Abstract
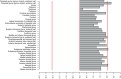
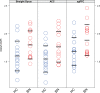
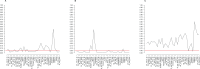
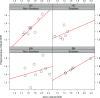
Bulimia nervosa (BN) shares central features with substance-related and addictive disorders. The metabotropic glutamate receptor subtype 5 (mGlu5) plays an important role in addiction. Based on similarities between binge eating and substance-related and addictive disorders, we investigated mGlu5 in vivo in 15 female subjects with BN and 15 matched controls. We measured mGlu5 distribution volume ratio (DVR) with positron emission tomography (PET) using [11 C]ABP688. In BN mGlu5 DVR was higher in the anterior cingulate cortex (ACC), subgenual prefrontal cortex, and straight gyrus (p < 0.05). In BN, higher mGlu5 DVR in various brain regions, including ACC, pallidum, putamen, and caudate, positively correlated with "maturity fears" as assessed using the Eating Disorder Inventory-2 (p < 0.05). In BN and controls, smokers had globally decreased mGlu5 DVR. We present the first evidence for increased mGlu5 DVR in BN. Our findings suggest that pharmacological agents inhibiting mGlu5 might have a therapeutic potential in BN.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

